Lung cancer is the malignant transformation Transformation Change brought about to an organism's genetic composition by unidirectional transfer (transfection; transduction, genetic; conjugation, genetic, etc.) and incorporation of foreign DNA into prokaryotic or eukaryotic cells by recombination of part or all of that DNA into the cell's genome. Bacteriology of lung tissue and the leading cause of cancer-related deaths. The majority of cases are associated with long-term smoking Smoking Willful or deliberate act of inhaling and exhaling smoke from burning substances or agents held by hand. Interstitial Lung Diseases. The disease is generally classified histologically as either small cell lung cancer or non-small cell lung cancer. Molecular profiling of the cancer provides further distinction of the tumor Tumor Inflammation's biological behavior, prognosis Prognosis A prediction of the probable outcome of a disease based on a individual's condition and the usual course of the disease as seen in similar situations. Non-Hodgkin Lymphomas, and treatment options. Symptoms include cough, dyspnea Dyspnea Dyspnea is the subjective sensation of breathing discomfort. Dyspnea is a normal manifestation of heavy physical or psychological exertion, but also may be caused by underlying conditions (both pulmonary and extrapulmonary). Dyspnea, weight loss Weight loss Decrease in existing body weight. Bariatric Surgery, and chest discomfort. Regional and metastatic spread cause additional symptoms and complications depending on the location and organ(s) affected. Related paraneoplastic syndromes Paraneoplastic syndromes Paraneoplastic syndromes are a heterogeneous group of disorders caused by an abnormal immune response to a neoplasm. The substances produced are not due to the direct effect of the tumor, such as metastasis, mass effect, or invasion. Antibodies, hormones, cytokines, and other substances are generated and affect multiple organ systems. Paraneoplastic Syndromes include hypercalcemia Hypercalcemia Hypercalcemia (serum calcium > 10.5 mg/dL) can result from various conditions, the majority of which are due to hyperparathyroidism and malignancy. Other causes include disorders leading to vitamin D elevation, granulomatous diseases, and the use of certain pharmacological agents. Symptoms vary depending on calcium levels and the onset of hypercalcemia. Hypercalcemia, hyponatremia Hyponatremia Hyponatremia is defined as a decreased serum sodium (sNa+) concentration less than 135 mmol/L. Serum sodium is the greatest contributor to plasma osmolality, which is very tightly controlled via antidiuretic hormone (ADH) release from the hypothalamus and by the thirst mechanism. Hyponatremia, Lambert-Eaton syndrome, Cushing's syndrome, polydermatomyositis, and dermatomyositis Dermatomyositis A subacute or chronic inflammatory disease of muscle and skin, marked by proximal muscle weakness and a characteristic skin rash. The illness occurs with approximately equal frequency in children and adults. The skin lesions usually take the form of a purplish rash (or less often an exfoliative dermatitis) involving the nose, cheeks, forehead, upper trunk, and arms. The disease is associated with a complement mediated intramuscular microangiopathy, leading to loss of capillaries, muscle ischemia, muscle-fiber necrosis, and perifascicular atrophy. The childhood form of this disease tends to evolve into a systemic vasculitis. Dermatomyositis may occur in association with malignant neoplasms. Paraneoplastic Syndromes. Definitive diagnosis and staging Staging Methods which attempt to express in replicable terms the extent of the neoplasm in the patient. Grading, Staging, and Metastasis are made by biopsy Biopsy Removal and pathologic examination of specimens from the living body. Ewing Sarcoma, genetic mutation Mutation Genetic mutations are errors in DNA that can cause protein misfolding and dysfunction. There are various types of mutations, including chromosomal, point, frameshift, and expansion mutations. Types of Mutations with biomarker testing, and imaging. Management is guided by the cancer stage and associated molecular profile. Lung cancer carries an overall poor prognosis Prognosis A prediction of the probable outcome of a disease based on a individual's condition and the usual course of the disease as seen in similar situations. Non-Hodgkin Lymphomas.
Last updated: Nov 27, 2024
Subtypes:
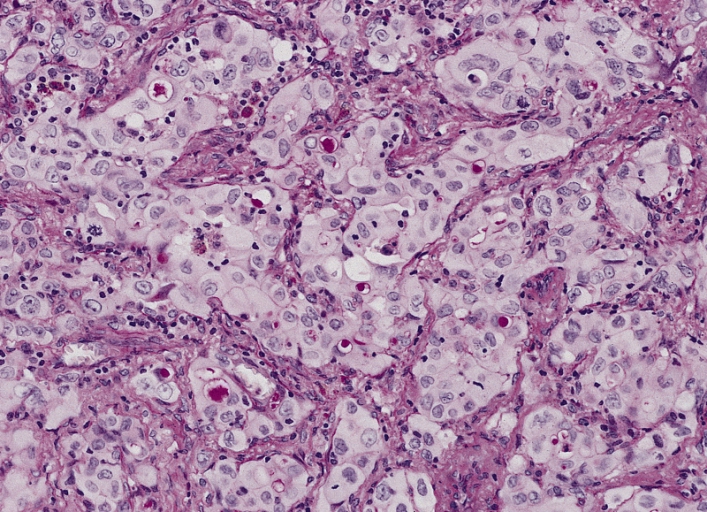
Adenocarcinoma of the lung:
A poorly differentiated (solid) adenocarcinoma showing many cells with mucin vacuoles
Subtypes:
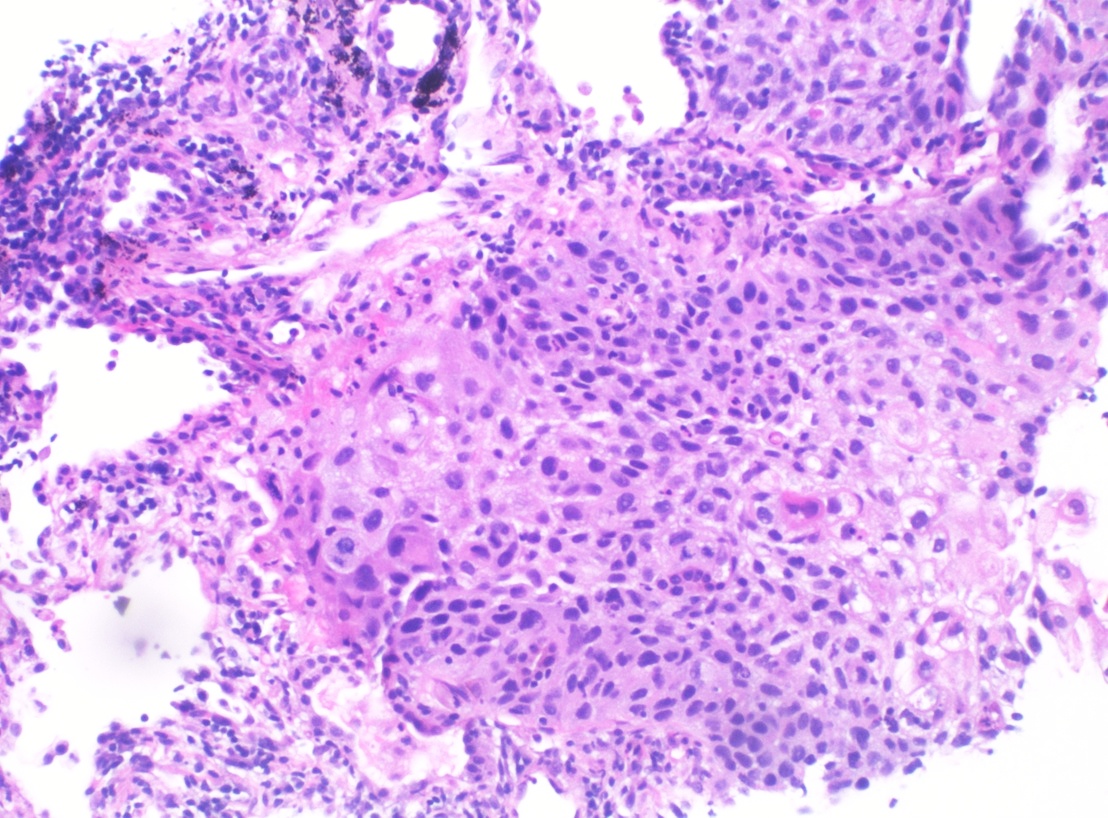
Squamous cell carcinoma on lung biopsy:
Histopathology of poorly differentiated SCC of the lung (H&E stain)
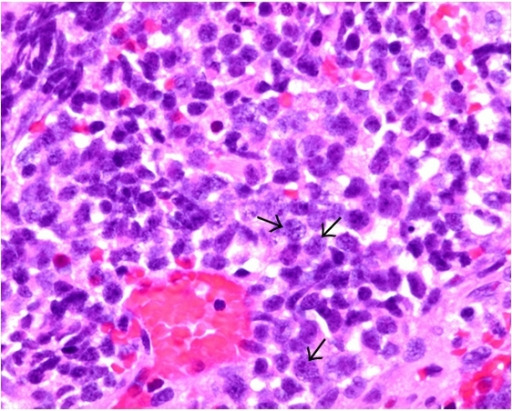
Small cell carcinoma:
The tumor cells (see arrows) exhibit features of small cell carcinoma, which include nuclear molding, high nuclear:cytoplasm ratio, and fine or salt-and-pepper chromatin-patterned nuclei on H&E staining.
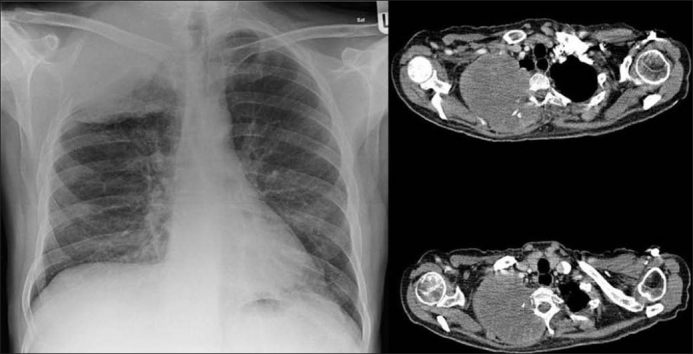
Pancoast tumor:
Chest X-ray on the left shows a right lung neoplasm invading the rib and thoracic wall. Computed tomography on the right shows the tumor invading the ribs, thoracic wall, and thoracic vertebrae.
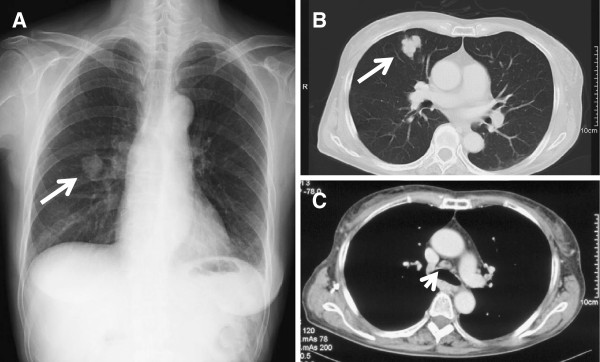
Chest radiograph and CT scan at diagnosis:
(A): Chest radiograph showing a shadow of a mass in the right middle zone (arrow).
(B, C): Conventional chest CT showing a 30-mm solitary mass in S5 of the right lung (arrow) and mediastinal lymphadenopathy (arrowhead).
In addition to CT, the following modalities are used to determine the extent of disease for staging Staging Methods which attempt to express in replicable terms the extent of the neoplasm in the patient. Grading, Staging, and Metastasis purposes:
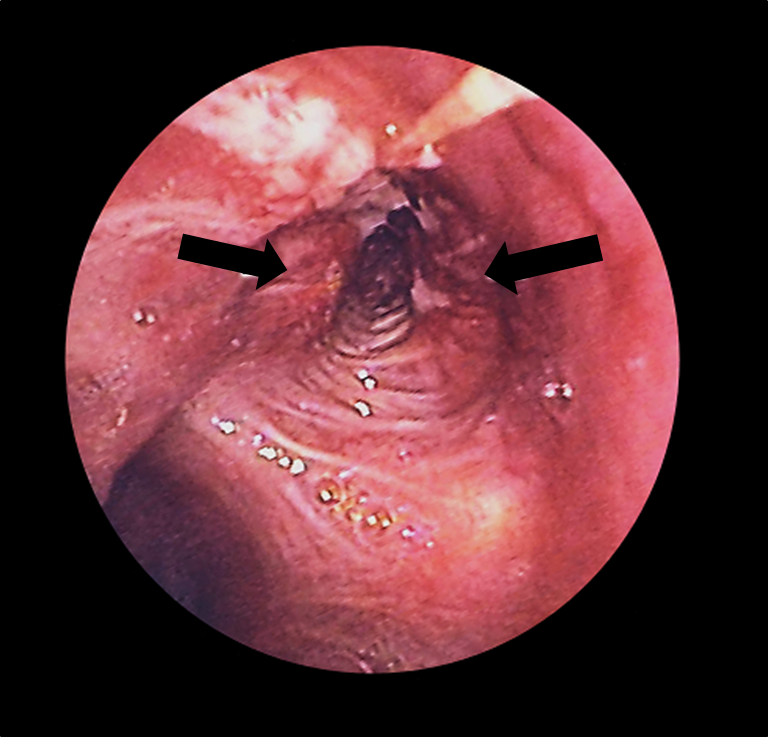
Bronchoscopy:
Lung cancer (arrows) in the left bronchus as seen with a bronchoscope
The following tests are possible findings for lung cancer:
The following are the 8th-edition staging Staging Methods which attempt to express in replicable terms the extent of the neoplasm in the patient. Grading, Staging, and Metastasis guidelines from the International Association for the Study of Lung Cancer:
| Stage | Description |
|---|---|
| Tx | Primary tumor Tumor Inflammation cannot be assessed. |
| T0 | No evidence of a primary tumor Tumor Inflammation |
| Tis | Carcinoma in situ |
| T1 |
Tumor
Tumor
Inflammation ≤ 3 cm, surrounded by lung or
visceral pleura
Visceral pleura
Pleura: Anatomy, without invasion of the main bronchus:
|
| T2 |
Tumor
Tumor
Inflammation > 3 cm but ≤ 5 cm, or:
|
| T3 T3 A T3 thyroid hormone normally synthesized and secreted by the thyroid gland in much smaller quantities than thyroxine (T4). Most T3 is derived from peripheral monodeiodination of T4 at the 5′ position of the outer ring of the iodothyronine nucleus. The hormone finally delivered and used by the tissues is mainly t3. Thyroid Hormones | Tumor Tumor Inflammation > 5 cm but ≤ 7 cm, or associated tumor Tumor Inflammation nodules in the same lobe as the primary tumor Tumor Inflammation, or direct invasion of the chest wall Chest wall The chest wall consists of skin, fat, muscles, bones, and cartilage. The bony structure of the chest wall is composed of the ribs, sternum, and thoracic vertebrae. The chest wall serves as armor for the vital intrathoracic organs and provides the stability necessary for the movement of the shoulders and arms. Chest Wall: Anatomy, phrenic nerve Phrenic nerve The motor nerve of the diaphragm. The phrenic nerve fibers originate in the cervical spinal column (mostly C4) and travel through the cervical plexus to the diaphragm. Diaphragm: Anatomy, or parietal Parietal One of a pair of irregularly shaped quadrilateral bones situated between the frontal bone and occipital bone, which together form the sides of the cranium. Skull: Anatomy pericardium Pericardium A conical fibroserous sac surrounding the heart and the roots of the great vessels (aorta; venae cavae; pulmonary artery). Pericardium consists of two sacs: the outer fibrous pericardium and the inner serous pericardium. The latter consists of an outer parietal layer facing the fibrous pericardium, and an inner visceral layer (epicardium) resting next to the heart, and a pericardial cavity between these two layers. Heart: Anatomy |
| T4 T4 The major hormone derived from the thyroid gland. Thyroxine is synthesized via the iodination of tyrosines (monoiodotyrosine) and the coupling of iodotyrosines (diiodotyrosine) in the thyroglobulin. Thyroxine is released from thyroglobulin by proteolysis and secreted into the blood. Thyroxine is peripherally deiodinated to form triiodothyronine which exerts a broad spectrum of stimulatory effects on cell metabolism. Thyroid Hormones | Tumor Tumor Inflammation > 7 cm, or associated tumor Tumor Inflammation nodules in a different ipsilateral lobe from the primary tumor Tumor Inflammation, or invasion into the diaphragm Diaphragm The diaphragm is a large, dome-shaped muscle that separates the thoracic cavity from the abdominal cavity. The diaphragm consists of muscle fibers and a large central tendon, which is divided into right and left parts. As the primary muscle of inspiration, the diaphragm contributes 75% of the total inspiratory muscle force. Diaphragm: Anatomy, mediastinum Mediastinum The mediastinum is the thoracic area between the 2 pleural cavities. The mediastinum contains vital structures of the circulatory, respiratory, digestive, and nervous systems including the heart and esophagus, and major thoracic vessels. Mediastinum and Great Vessels: Anatomy, heart, great vessels, trachea Trachea The trachea is a tubular structure that forms part of the lower respiratory tract. The trachea is continuous superiorly with the larynx and inferiorly becomes the bronchial tree within the lungs. The trachea consists of a support frame of semicircular, or C-shaped, rings made out of hyaline cartilage and reinforced by collagenous connective tissue. Trachea: Anatomy, esophagus Esophagus The esophagus is a muscular tube-shaped organ of around 25 centimeters in length that connects the pharynx to the stomach. The organ extends from approximately the 6th cervical vertebra to the 11th thoracic vertebra and can be divided grossly into 3 parts: the cervical part, the thoracic part, and the abdominal part. Esophagus: Anatomy, recurrent laryngeal nerve, vertebral body Vertebral body Main portion of the vertebra which bears majority of the weight. Vertebral Column: Anatomy, or carina |
| Stage | Description |
|---|---|
| Nx | Unable to assess regional lymph nodes Lymph Nodes They are oval or bean shaped bodies (1 – 30 mm in diameter) located along the lymphatic system. Lymphatic Drainage System: Anatomy |
| N0 | No regional lymph Lymph The interstitial fluid that is in the lymphatic system. Secondary Lymphatic Organs node metastasis Metastasis The transfer of a neoplasm from one organ or part of the body to another remote from the primary site. Grading, Staging, and Metastasis |
| N1 | Ipsilateral peribronchial and/or hilar lymph nodes Lymph Nodes They are oval or bean shaped bodies (1 – 30 mm in diameter) located along the lymphatic system. Lymphatic Drainage System: Anatomy and intrapulmonary node involvement |
| N2 | Ipsilateral mediastinal and/or subcarinal lymph Lymph The interstitial fluid that is in the lymphatic system. Secondary Lymphatic Organs node involvement |
| N3 | Contralateral mediastinal, contralateral hilar, ipsilateral or contralateral scalene, or supraclavicular lymph Lymph The interstitial fluid that is in the lymphatic system. Secondary Lymphatic Organs node involvement |
| Stage | Description |
|---|---|
| M0 | No distant metastasis Metastasis The transfer of a neoplasm from one organ or part of the body to another remote from the primary site. Grading, Staging, and Metastasis |
| M1 | Distant
metastasis
Metastasis
The transfer of a neoplasm from one organ or part of the body to another remote from the primary site.
Grading, Staging, and Metastasis:
|
| Stage grouping | TNM stage |
|---|---|
| Occult |
|
| 0 |
|
| I |
|
| II |
|
| III |
|
| IV |
|
| Stage (SCLC) | Description |
|---|---|
| Limited disease |
|
| Extensive disease |
|
Surgery:
Chemotherapy Chemotherapy Osteosarcoma:
Radiation Radiation Emission or propagation of acoustic waves (sound), electromagnetic energy waves (such as light; radio waves; gamma rays; or x-rays), or a stream of subatomic particles (such as electrons; neutrons; protons; or alpha particles). Osteosarcoma therapy:
Targeted therapies:
Immunotherapy:
| Stage (NSCLC) | Treatment approach |
|---|---|
| Stage I–II |
|
| Stage III |
|
| Stage IV* |
|
| Stage (SCLC) | Description |
|---|---|
| Limited disease |
|
| Extensive disease* |
|
Risk-factor modification is more effective than screening Screening Preoperative Care in reducing lung cancer death.