Hypercoagulable states (also referred to as thrombophilias) are a group of hematologic diseases defined by an increased risk of clot formation (i.e., thrombosis Thrombosis Formation and development of a thrombus or blood clot in the blood vessel. Epidemic Typhus) due to either an increase in procoagulants, a decrease in anticoagulants Anticoagulants Anticoagulants are drugs that retard or interrupt the coagulation cascade. The primary classes of available anticoagulants include heparins, vitamin K-dependent antagonists (e.g., warfarin), direct thrombin inhibitors, and factor Xa inhibitors. Anticoagulants, or a decrease in fibrinolysis. There are both inherited and acquired causes, with factor V Factor V Heat- and storage-labile plasma glycoprotein which accelerates the conversion of prothrombin to thrombin in blood coagulation. Factor V accomplishes this by forming a complex with factor Xa, phospholipid, and calcium (prothrombinase complex). Hemostasis Leiden being the most common inherited cause. Clinically, hypercoagulable states present with thrombotic events, which cause vessel occlusion and can lead to organ damage. Thrombotic disorders can be fatal if not treated, and management usually involves anticoagulants Anticoagulants Anticoagulants are drugs that retard or interrupt the coagulation cascade. The primary classes of available anticoagulants include heparins, vitamin K-dependent antagonists (e.g., warfarin), direct thrombin inhibitors, and factor Xa inhibitors. Anticoagulants.
Last updated: Mar 24, 2025
Hypercoagulability, also referred to as a thrombophilia, refers to the increased tendency for blood to form clots, known as thrombi. Hypercoagulable states can be inherited or acquired.
Prevalence Prevalence The total number of cases of a given disease in a specified population at a designated time. It is differentiated from incidence, which refers to the number of new cases in the population at a given time. Measures of Disease Frequency of inherited thrombophilias:
| Condition | Prevalence Prevalence The total number of cases of a given disease in a specified population at a designated time. It is differentiated from incidence, which refers to the number of new cases in the population at a given time. Measures of Disease Frequency | Risk of VTE |
|---|---|---|
| Factor V Factor V Heat- and storage-labile plasma glycoprotein which accelerates the conversion of prothrombin to thrombin in blood coagulation. Factor V accomplishes this by forming a complex with factor Xa, phospholipid, and calcium (prothrombinase complex). Hemostasis Leiden | 3%–7% | 4.3% |
| Prothrombin Prothrombin A plasma protein that is the inactive precursor of thrombin. It is converted to thrombin by a prothrombin activator complex consisting of factor Xa, factor V, phospholipid, and calcium ions. Hemostasis G20210A mutation Mutation Genetic mutations are errors in DNA that can cause protein misfolding and dysfunction. There are various types of mutations, including chromosomal, point, frameshift, and expansion mutations. Types of Mutations | 1%–3% | 1.9% |
| Protein C deficiency (heterozygous) | 0.02%–0.05% | 11.3% |
| Protein S Protein S Protein S augments the activity of protein C. Hemostasis deficiency (heterozygous) | 0.01% | 32.4% |
| Antithrombin deficiency Antithrombin deficiency An absence or reduced level of antithrombin III leading to an increased risk for thrombosis. Budd-Chiari Syndrome | 0.02%–0.04% | 17.5% |
Most common acquired hypercoagulable states:
Clinical impact:
Thrombotic events occur under 3 primary conditions, which make up Virchow’s triad Virchow’s triad Deep Vein Thrombosis. These 3 conditions are:
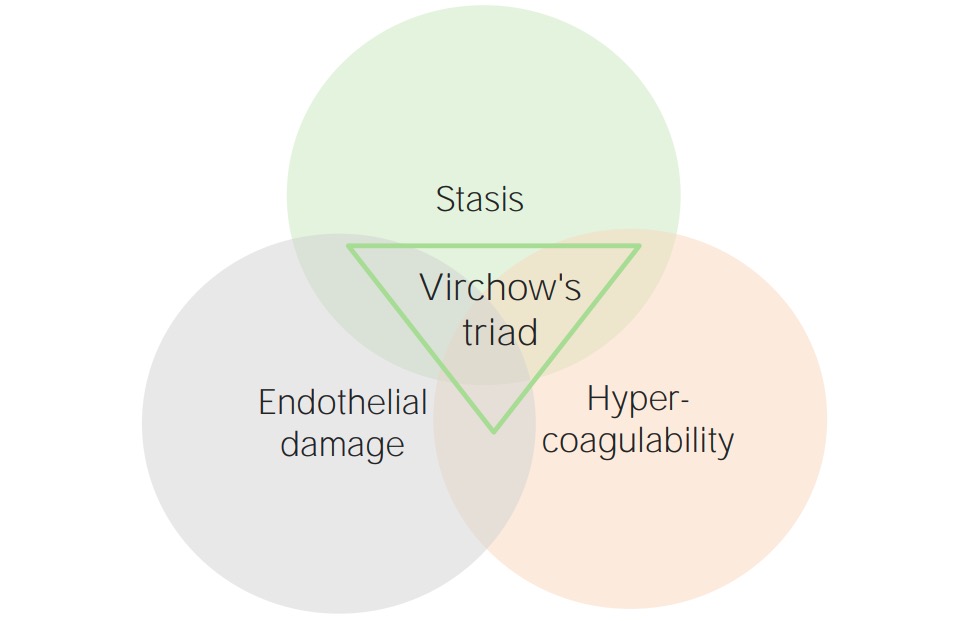
Virchow’s triad
Image by Lecturio.Hypercoagulable states may be primary (inherited) or secondary (acquired).
| Condition | Inheritance pattern | Pathophysiology |
|---|---|---|
| Factor V Factor V Heat- and storage-labile plasma glycoprotein which accelerates the conversion of prothrombin to thrombin in blood coagulation. Factor V accomplishes this by forming a complex with factor Xa, phospholipid, and calcium (prothrombinase complex). Hemostasis Leiden | Autosomal dominant Autosomal dominant Autosomal inheritance, both dominant and recessive, refers to the transmission of genes from the 22 autosomal chromosomes. Autosomal dominant diseases are expressed when only 1 copy of the dominant allele is inherited. Autosomal Recessive and Autosomal Dominant Inheritance with incomplete penetrance Penetrance The percent frequency with which a dominant or homozygous recessive gene or gene combination manifests itself in the phenotype of the carriers. Familial Juvenile Polyposis |
|
| Prothrombin Prothrombin A plasma protein that is the inactive precursor of thrombin. It is converted to thrombin by a prothrombin activator complex consisting of factor Xa, factor V, phospholipid, and calcium ions. Hemostasis G20210A (also known as factor II mutation Mutation Genetic mutations are errors in DNA that can cause protein misfolding and dysfunction. There are various types of mutations, including chromosomal, point, frameshift, and expansion mutations. Types of Mutations) | Autosomal dominant Autosomal dominant Autosomal inheritance, both dominant and recessive, refers to the transmission of genes from the 22 autosomal chromosomes. Autosomal dominant diseases are expressed when only 1 copy of the dominant allele is inherited. Autosomal Recessive and Autosomal Dominant Inheritance |
|
| Antithrombin deficiency Antithrombin deficiency An absence or reduced level of antithrombin III leading to an increased risk for thrombosis. Budd-Chiari Syndrome | Autosomal dominant Autosomal dominant Autosomal inheritance, both dominant and recessive, refers to the transmission of genes from the 22 autosomal chromosomes. Autosomal dominant diseases are expressed when only 1 copy of the dominant allele is inherited. Autosomal Recessive and Autosomal Dominant Inheritance |
|
| Protein C or S deficiency | Autosomal dominant Autosomal dominant Autosomal inheritance, both dominant and recessive, refers to the transmission of genes from the 22 autosomal chromosomes. Autosomal dominant diseases are expressed when only 1 copy of the dominant allele is inherited. Autosomal Recessive and Autosomal Dominant Inheritance (rare; can be recessive) |
|
| Sticky platelet syndrome | Autosomal dominant Autosomal dominant Autosomal inheritance, both dominant and recessive, refers to the transmission of genes from the 22 autosomal chromosomes. Autosomal dominant diseases are expressed when only 1 copy of the dominant allele is inherited. Autosomal Recessive and Autosomal Dominant Inheritance |
|
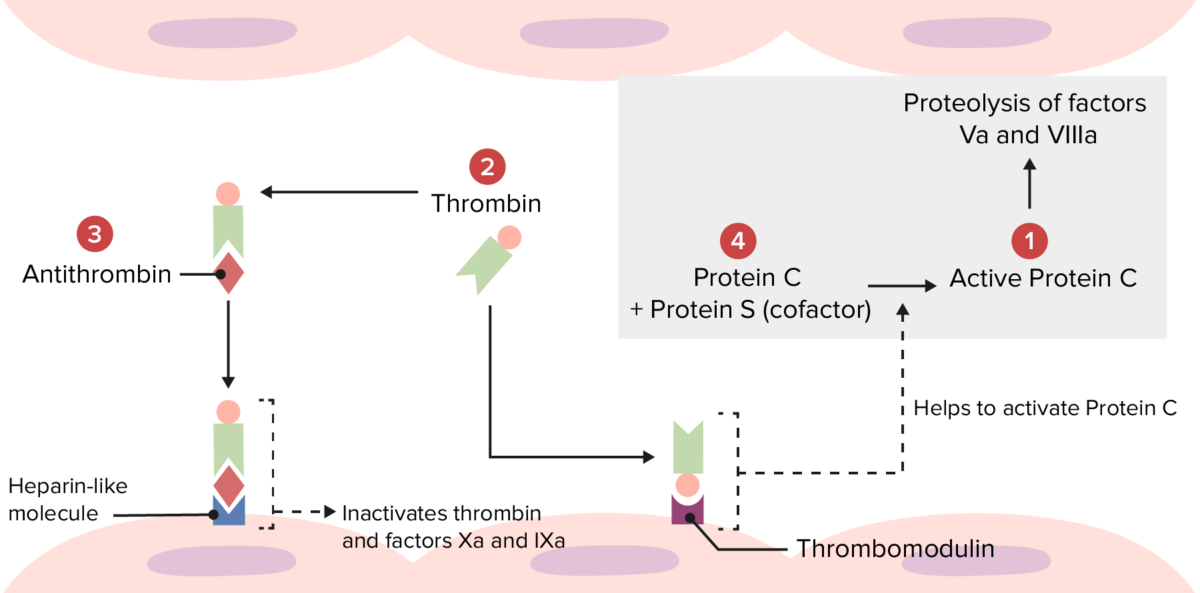
Overview of the physiologic thrombolytic pathway:
Several hypercoagulable disorders occur because of abnormalities at the following locations.
Mutations at 1: Factor V Leiden makes factor Va resistant to degradation by activated protein C.
Mutations at 2: Prothrombin mutation produces an increased amount of prothrombin, leading to excess thrombin formation.
Mutations at 3: Antithrombin deficiency leads to reduced inactivation of thrombin and factors Xa and IXa.
Mutations at 4: Protein C or S deficiency leads to reduced inactivation of factors Va and VIIIa.
Many other conditions and states increase thrombotic risk by affecting components of Virchow’s triad Virchow’s triad Deep Vein Thrombosis (typically, stasis, endothelial injury, or both) in ways that promote thrombosis Thrombosis Formation and development of a thrombus or blood clot in the blood vessel. Epidemic Typhus. These conditions include:
The primary clinical presentation of a hypercoagulable state will be a thrombotic event or an asymptomatic family member of a patient with a known primary hypercoagulable condition presenting for evaluation.
Pitting edema with swelling of the right leg due to a deep venous thrombosis
Image: “Pitting oedema of right leg” by Department of medicine (ward 45), the National hospital of Sri Lanka, (Regent Street), Colombo, (00800), Sri Lanka. License: CC BY 2.0Factor V Factor V Heat- and storage-labile plasma glycoprotein which accelerates the conversion of prothrombin to thrombin in blood coagulation. Factor V accomplishes this by forming a complex with factor Xa, phospholipid, and calcium (prothrombinase complex). Hemostasis Leiden and prothrombin Prothrombin A plasma protein that is the inactive precursor of thrombin. It is converted to thrombin by a prothrombin activator complex consisting of factor Xa, factor V, phospholipid, and calcium ions. Hemostasis G20210A mutations do not have any unique presentations beyond recurrent thrombotic events. Several specific findings may be noted in:
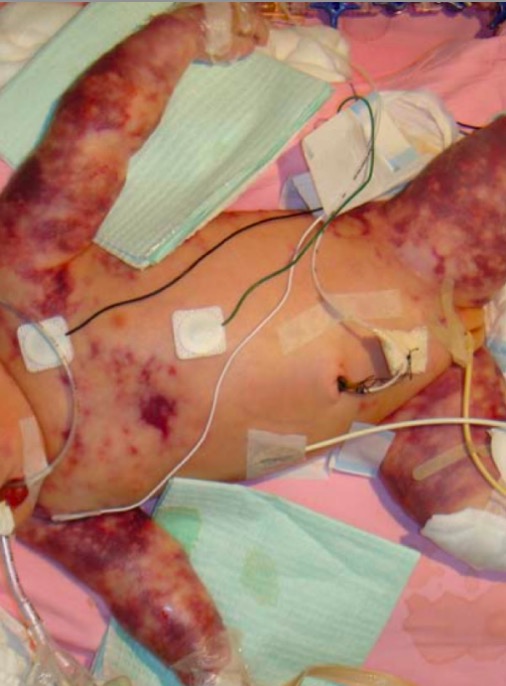
Neonatal purpura fulminans
Image: “Purupura fulminans” by Department of Paediatrics, The Chinese University of Hong Kong, Prince of Wales Hospital, Shatin, Hong Kong. License: CC BY 2.0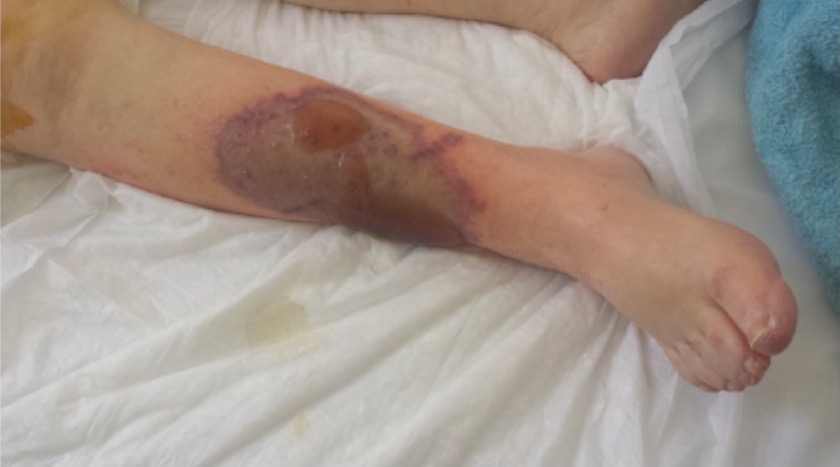
Warfarin-induced skin necrosis
Image: “Patient’s right leg” by 1st Department of Surgery, Vascular Surgery Unit, Laikon General Hospital, Medical School of Athens, Agiou Thoma 17, 11527 Athens, Greece. License: CC BY 4.0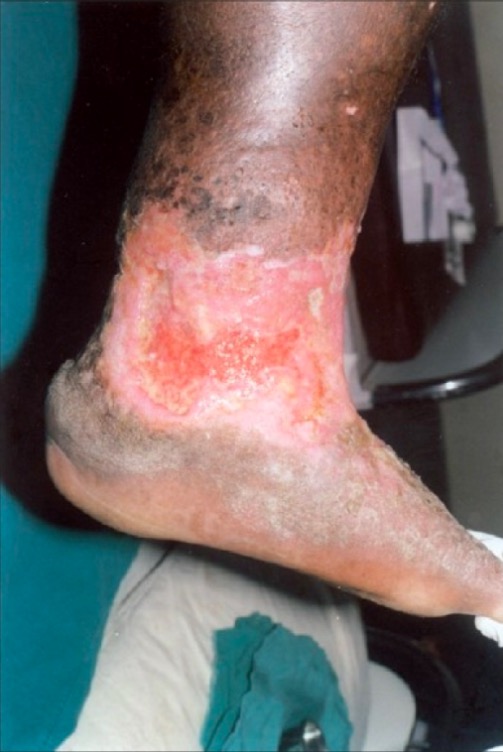
Irregular, nonhealing venous stasis ulcer on the lower leg and ankle, with unhealthy granulation tissue:
There is also surrounding lipodermatosclerosis, stasis dermatitis, and brown skin pigmentation, all characteristic of chronic venous insufficiency.
These studies should be performed in most patients Patients Individuals participating in the health care system for the purpose of receiving therapeutic, diagnostic, or preventive procedures. Clinician–Patient Relationship with suspected thrombotic events.
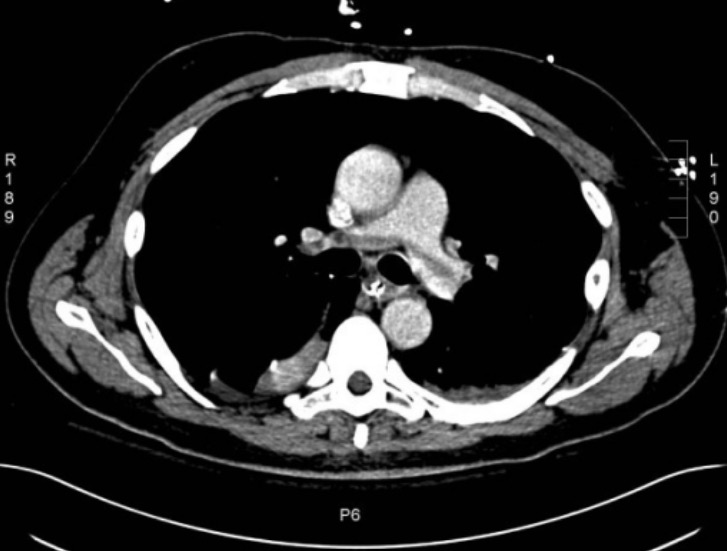
CT angiogram of the chest showing a saddle pulmonary embolus
Image: “Large saddle pulmonary embolism” by Rhode Island Hospital, Brown University School of Medicine, 2 Dudley Street, Providence, RI, USA. License: CC BY 2.0Consider ordering additional specific tests to screen for an inherited thrombophilia if patients Patients Individuals participating in the health care system for the purpose of receiving therapeutic, diagnostic, or preventive procedures. Clinician–Patient Relationship meet any of the following criteria:
If patients Patients Individuals participating in the health care system for the purpose of receiving therapeutic, diagnostic, or preventive procedures. Clinician–Patient Relationship meet any of the above criteria for a thrombophilia workup, the following tests can be ordered to screen for specific inherited thrombophilias:
Note: Acute thrombosis Thrombosis Formation and development of a thrombus or blood clot in the blood vessel. Epidemic Typhus and/or anticoagulants Anticoagulants Anticoagulants are drugs that retard or interrupt the coagulation cascade. The primary classes of available anticoagulants include heparins, vitamin K-dependent antagonists (e.g., warfarin), direct thrombin inhibitors, and factor Xa inhibitors. Anticoagulants may reduce plasma Plasma The residual portion of blood that is left after removal of blood cells by centrifugation without prior blood coagulation. Transfusion Products concentrations of antithrombin Antithrombin Endogenous factors and drugs that directly inhibit the action of thrombin, usually by blocking its enzymatic activity. They are distinguished from indirect thrombin inhibitors, such as heparin, which act by enhancing the inhibitory effects of antithrombins. Anticoagulants, protein C, and protein S Protein S Protein S augments the activity of protein C. Hemostasis.
In patients Patients Individuals participating in the health care system for the purpose of receiving therapeutic, diagnostic, or preventive procedures. Clinician–Patient Relationship with known inherited thrombophilias or who have risk factors for thrombosis Thrombosis Formation and development of a thrombus or blood clot in the blood vessel. Epidemic Typhus:
Anticoagulation Anticoagulation Pulmonary Hypertension Drugs is the mainstay of therapy for thrombotic events. Options for initial anticoagulation Anticoagulation Pulmonary Hypertension Drugs include:
Duration of therapy: