Immunoglobulins (Igs), also known as antibodies, are glycoprotein molecules produced by plasma Plasma The residual portion of blood that is left after removal of blood cells by centrifugation without prior blood coagulation. Transfusion Products cells that act in immune responses by recognizing and binding specific antigens. Antibodies undergo processes that improve antigen Antigen Substances that are recognized by the immune system and induce an immune reaction. Vaccination affinity and provide appropriate defense by class switching. The various Ig Ig X-linked Agammaglobulinemia classes are IgG IgG The major immunoglobulin isotype class in normal human serum. There are several isotype subclasses of igg, for example, igg1, igg2a, and igg2b. Hypersensitivity Pneumonitis (the most abundant), IgM, IgE, IgD, and IgA, which differ in their biologic features, structure, target specificity, and distribution. General functions include opsonization, neutralization of infectivity of the pathogens, cytotoxicity, and complement activation Complement Activation The sequential activation of serum complement proteins to create the complement membrane attack complex. Factors initiating complement activation include antigen-antibody complexes, microbial antigens, or cell surface polysaccharides. Systemic Lupus Erythematosus. Specific classes have unique defensive mechanisms.
Last updated: Dec 19, 2022
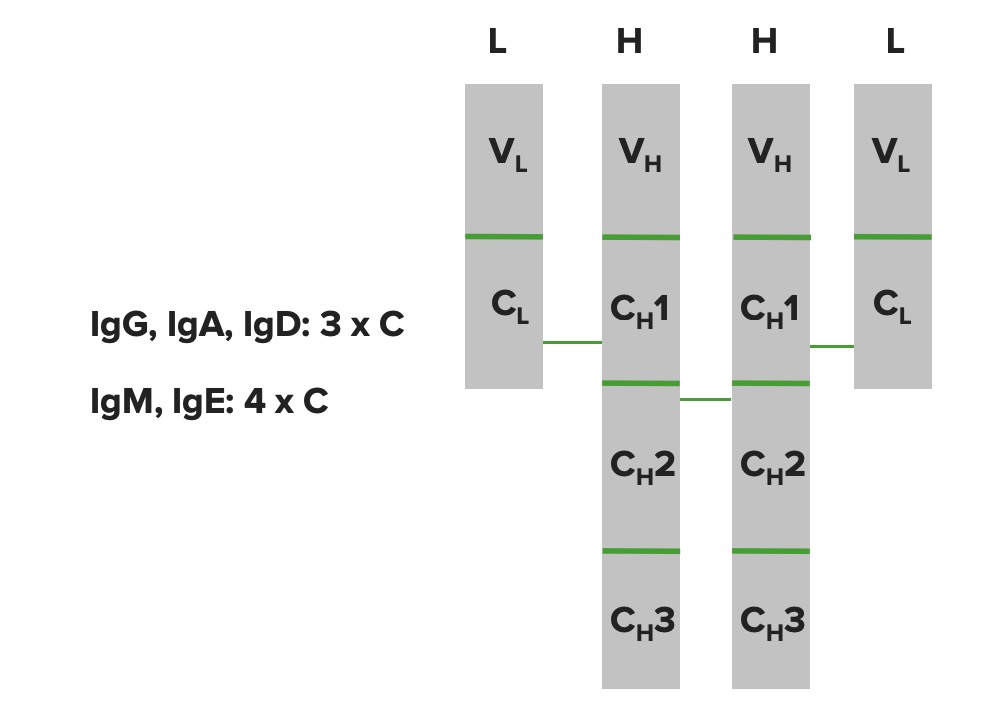
Immunoglobulin domains:
The heavy chains and light chains are folded up into domain type structures. The light chain has 1 variable domain and 1 constant domain. The heavy chain has 1 variable domain but has different constant domains depending on the Ig molecule (IgG, IgA, and IgD have 3 constant domains, whereas IgM and IgE have 4 constant domains).
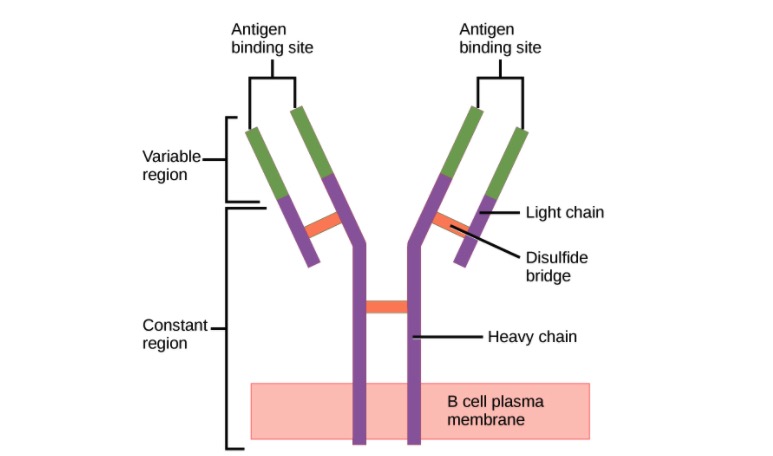
Structure of the antibody (regions):
Antibody has a unique variable region (formed by heavy and light chains) capable of binding a different antigen and a constant region (formed by heavy chains).
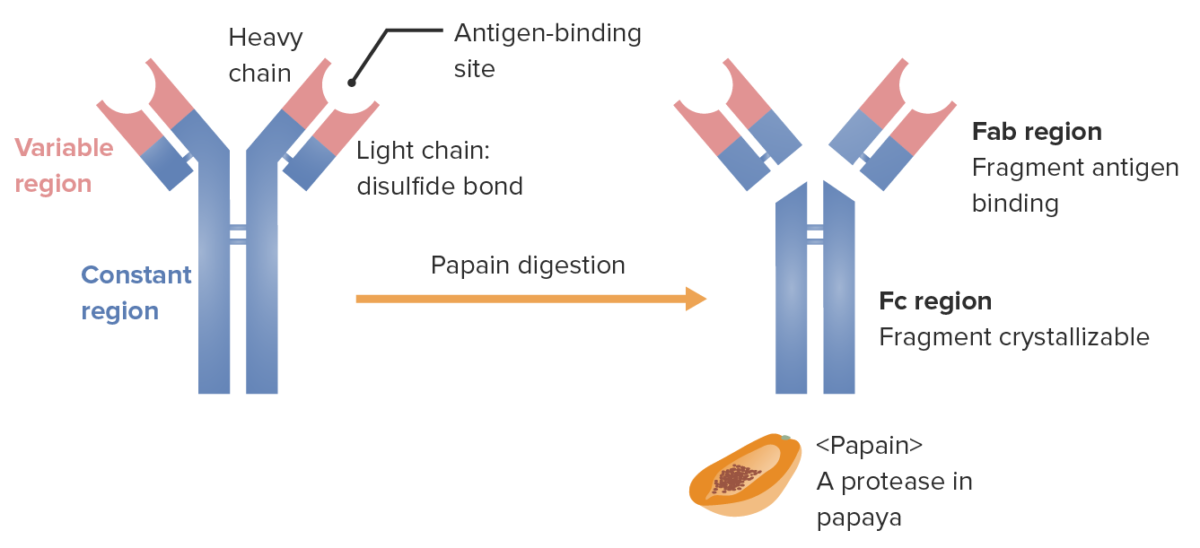
Fragments of the Ig (determined by location where the enzyme papain splits the Ig):
Fab (fragment antigen-binding) contains the variable regions (red) and parts of the constant region (blue) of both heavy and light chains. Fc (fragment crystallizable) contains the remaining part (tail) of the antibody (constant region of the heavy chain only).
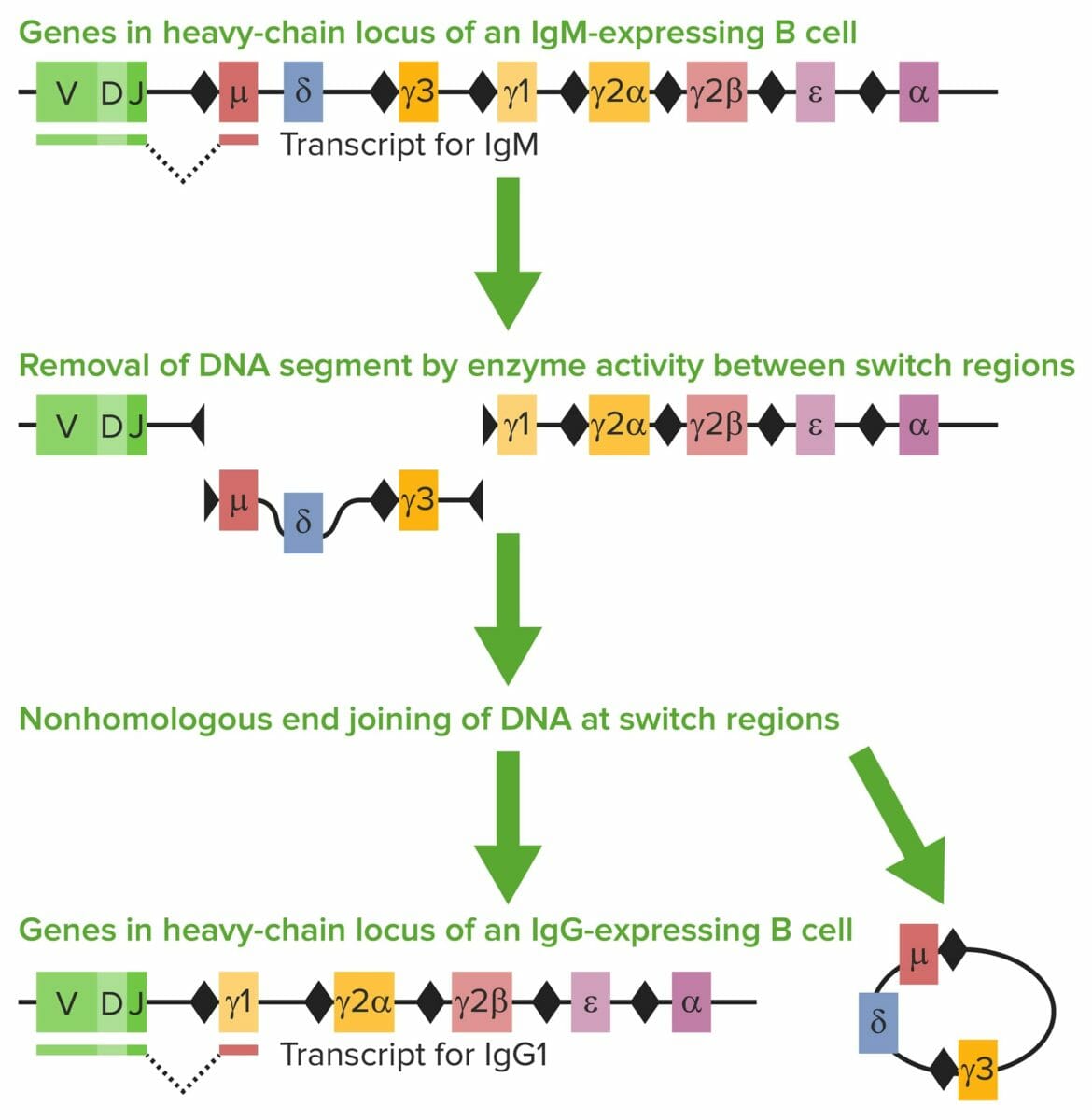
Class-switch recombination (CSR):
The heavy chain has different gene segments: variable region (V), diversity region (D), joining region (J), and constant (C) region.
The heavy-chain C region determines the Ig class/isotype. When antigens are encountered, mature IgM-positive B cells undergo CSR. Exons encoding the constant coding gene segment (Cμ) of IgH are excised. Repetitive areas of DNA, switch regions (the black diamonds), are present.
Switch regions guide enzymes (e.g., activation-induced cytidine deaminase (AICDA)) to where to create DNA double-stranded breaks (DSBs) and where the VDJ segment and the new constant region are joined by a repair enzyme. The Cμ is replaced with a new constant gene segment (e.g., Cγ, Cε, or Cα). In the image, Cγ1 attaches to the VDJ segment and creates IgG1.
Antibodies that are created have important properties (diversity and specificity) that are essential in the immune response.
Unique mechanisms creating antibody diversity Antibody Diversity The phenomenon of immense variability characteristic of antibodies. It enables the immune system to react specifically against the essentially unlimited kinds of antigens it encounters. Antibody diversity is accounted for by three main theories: (1) the germ line theory, which holds that each antibody-producing cell has genes coding for all possible antibody specificities, but expresses only the one stimulated by antigen; (2) the somatic mutation theory, which holds that antibody-producing cells contain only a few genes, which produce antibody diversity by mutation; and (3) the gene rearrangement theory, which holds that antibody diversity is generated by the rearrangement of immunoglobulin variable region gene segments during the differentiation of the antibody-producing cells. B cells: Types and Functions include:
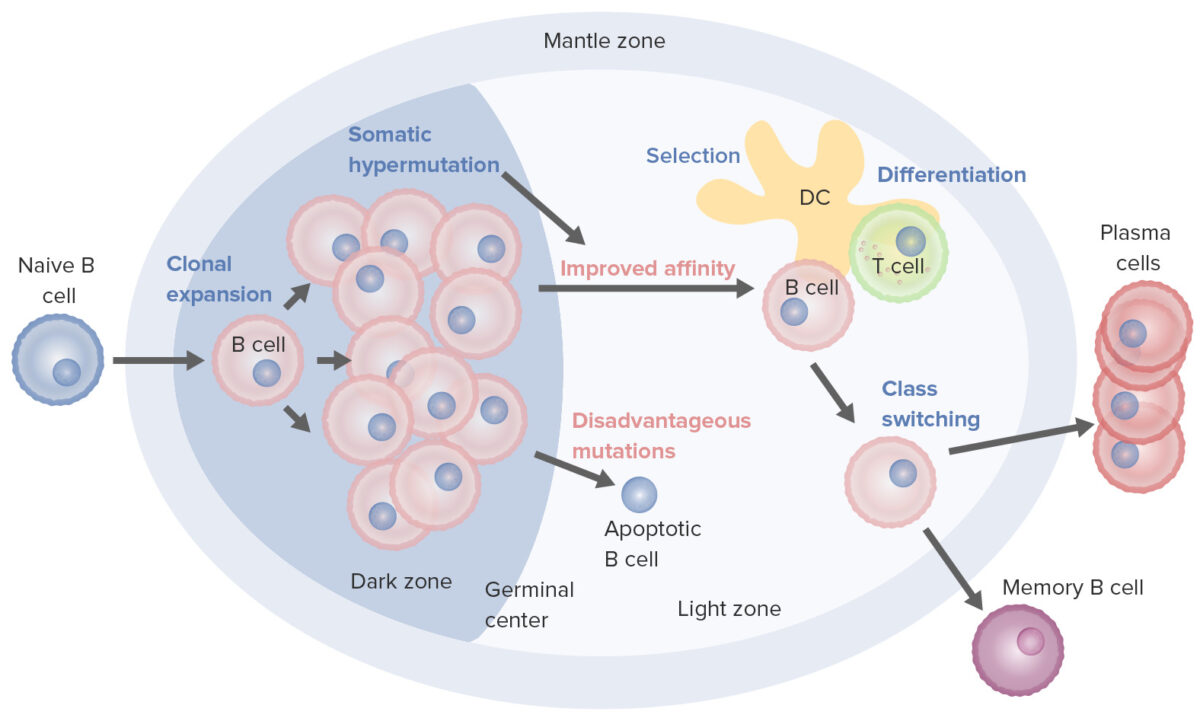
B-cell activation and maturation processes taking place in the germinal center:
On activation, the B cell moves from the mantle zone and enters the germinal center. B-cell proliferation (clonal expansion) takes place, and antibody affinity to the antigen is enhanced through the process of somatic hypermutation. Repeated cycles of proliferation and hypermutation fine-tune the B-cell receptor. However, not all B cells continue to differentiate, especially if affinity is weak. Apoptosis follows if the antigen–antibody binding is not optimized. B cells with strong affinity survive (selection), with the help of survival signals from follicular dendritic cells and T cells. These selected B cells move on to class switching and differentiation into plasma cells or memory cells.
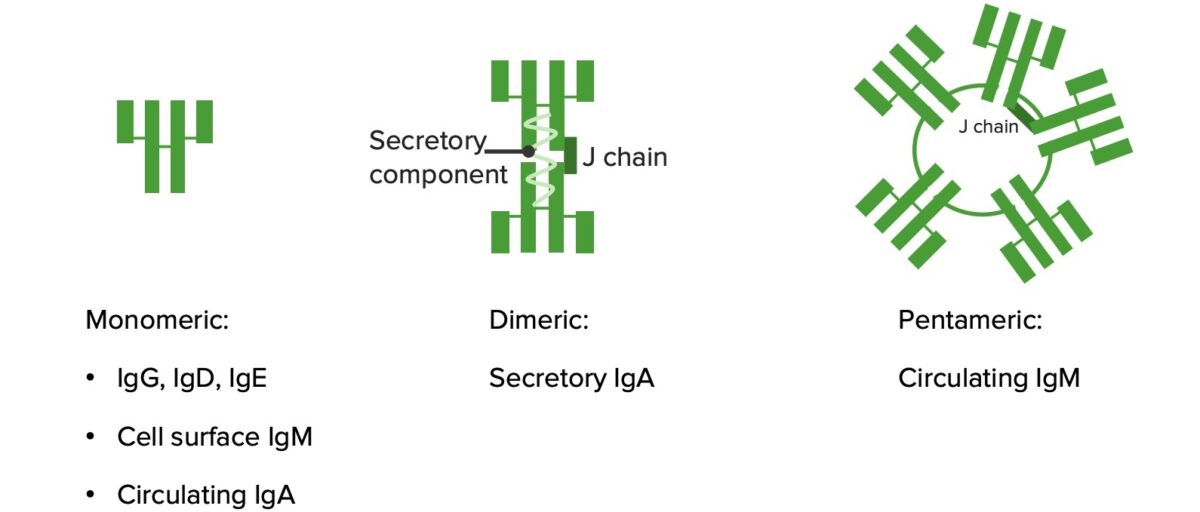
Monomers and polymers:
IgG, IgD, and IgE are monomers (structure illustrated on the leftmost image). IgA becomes a dimer in mucous secretions. IgM forms a pentamer.
Image by Lecturio.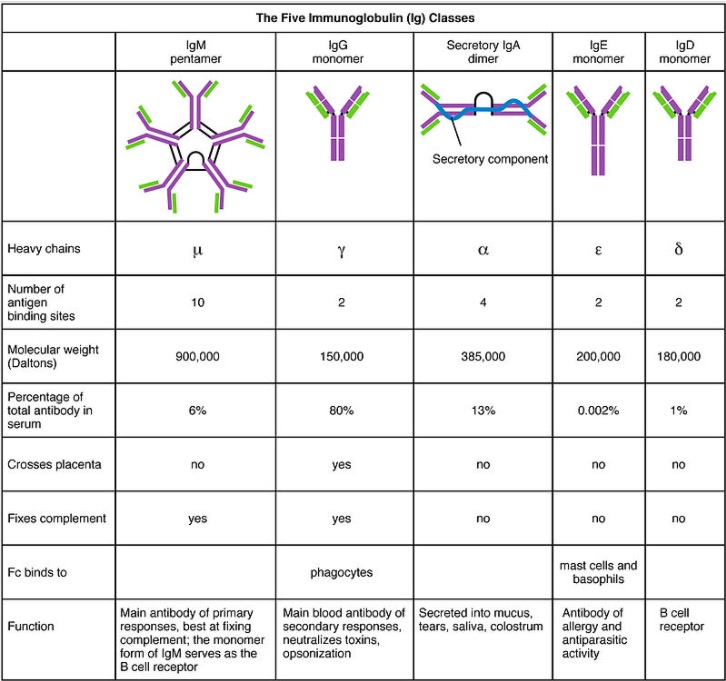
The 5 Ig classes, their structures, and their characteristics
Image: “Five immunoglobulin classes” by OpenStax College. License: CC BY 3.0