T cells, also called T lymphocytes Lymphocytes Lymphocytes are heterogeneous WBCs involved in immune response. Lymphocytes develop from the bone marrow, starting from hematopoietic stem cells (HSCs) and progressing to common lymphoid progenitors (CLPs). B and T lymphocytes and natural killer (NK) cells arise from the lineage. Lymphocytes: Histology, are important components of the adaptive immune system Immune system The body's defense mechanism against foreign organisms or substances and deviant native cells. It includes the humoral immune response and the cell-mediated response and consists of a complex of interrelated cellular, molecular, and genetic components. Primary Lymphatic Organs. Production starts from the hematopoietic stem cells Hematopoietic stem cells Progenitor cells from which all blood cells derived. They are found primarily in the bone marrow and also in small numbers in the peripheral blood. Bone Marrow: Composition and Hematopoiesis in the bone marrow Bone marrow The soft tissue filling the cavities of bones. Bone marrow exists in two types, yellow and red. Yellow marrow is found in the large cavities of large bones and consists mostly of fat cells and a few primitive blood cells. Red marrow is a hematopoietic tissue and is the site of production of erythrocytes and granular leukocytes. Bone marrow is made up of a framework of connective tissue containing branching fibers with the frame being filled with marrow cells. Bone Marrow: Composition and Hematopoiesis, from which T-cell progenitor cells arise. These cells migrate to the thymus Thymus A single, unpaired primary lymphoid organ situated in the mediastinum, extending superiorly into the neck to the lower edge of the thyroid gland and inferiorly to the fourth costal cartilage. It is necessary for normal development of immunologic function early in life. By puberty, it begins to involute and much of the tissue is replaced by fat. Lymphatic Drainage System: Anatomy for further maturation. A functional mature T cell develops from a step-by-step process creating a T-cell receptor Receptor Receptors are proteins located either on the surface of or within a cell that can bind to signaling molecules known as ligands (e.g., hormones) and cause some type of response within the cell. Receptors ( TCR TCR Molecules on the surface of T-lymphocytes that recognize and combine with antigens. The receptors are non-covalently associated with a complex of several polypeptides collectively called CD3 antigens. Recognition of foreign antigen and the major histocompatibility complex is accomplished by a single heterodimeric antigen-receptor structure, composed of either alpha-beta or gamma-delta chains. Adaptive Cell-mediated Immunity) complex, selecting T cells with appropriate affinity to self-antigens associated with major histocompatibility molecules (positive selection Selection Lymphocyte activation by a specific antigen thus triggering clonal expansion of lymphocytes already capable of mounting an immune response to the antigen. B cells: Types and Functions), and expressing either CD4 or CD8. In this series, cells predisposed to autoimmunity Autoimmunity Autoimmunity is a pathologic immune response toward self-antigens, resulting from a combination of factors: immunologic, genetic, and environmental. The immune system is equipped with self-tolerance, allowing immune cells such as T cells and B cells to recognize self-antigens and to not mount a reaction against them. Defects in this mechanism, along with environmental triggers (such as infections) and genetic susceptibility factors (most notable of which are the HLA genes) can lead to autoimmune diseases. Autoimmunity undergo apoptosis Apoptosis A regulated cell death mechanism characterized by distinctive morphologic changes in the nucleus and cytoplasm, including the endonucleolytic cleavage of genomic DNA, at regularly spaced, internucleosomal sites, I.e., DNA fragmentation. It is genetically-programmed and serves as a balance to mitosis in regulating the size of animal tissues and in mediating pathologic processes associated with tumor growth. Ischemic Cell Damage (negative selection Selection Lymphocyte activation by a specific antigen thus triggering clonal expansion of lymphocytes already capable of mounting an immune response to the antigen. B cells: Types and Functions). When released from the thymus Thymus A single, unpaired primary lymphoid organ situated in the mediastinum, extending superiorly into the neck to the lower edge of the thyroid gland and inferiorly to the fourth costal cartilage. It is necessary for normal development of immunologic function early in life. By puberty, it begins to involute and much of the tissue is replaced by fat. Lymphatic Drainage System: Anatomy, the naive mature T cells travel to the secondary lymphoid organs Lymphoid organs A system of organs and tissues that process and transport immune cells and lymph. Primary Lymphatic Organs for activation. Two signals, an antigen-specific binding of TCR TCR Molecules on the surface of T-lymphocytes that recognize and combine with antigens. The receptors are non-covalently associated with a complex of several polypeptides collectively called CD3 antigens. Recognition of foreign antigen and the major histocompatibility complex is accomplished by a single heterodimeric antigen-receptor structure, composed of either alpha-beta or gamma-delta chains. Adaptive Cell-mediated Immunity and costimulation Costimulation Adaptive Cell-mediated Immunity, are required to be activated (ready to mount an immune response). In the case of CD8+ T cells, additional cytokine stimulation is necessary. Depending on the cytokines Cytokines Non-antibody proteins secreted by inflammatory leukocytes and some non-leukocytic cells, that act as intercellular mediators. They differ from classical hormones in that they are produced by a number of tissue or cell types rather than by specialized glands. They generally act locally in a paracrine or autocrine rather than endocrine manner. Adaptive Immune Response they are exposed to during antigen Antigen Substances that are recognized by the immune system and induce an immune reaction. Vaccination stimulation, the undifferentiated mature T cell (Th0) develops into cells with different functions: CD4+ become T helper (Th) cells and CD8+ become cytotoxic Cytotoxic Parvovirus B19, or cytolytic, cells. Th cells have other subtypes; the most characterized are Th1, Th2, Th17, follicular Th cells, and regulatory T cells Regulatory T cells Autoimmunity. Other types include natural killer T cells and memory T cells Memory T cells Adaptive Cell-mediated Immunity. These mature differentiated T cells ensure effective surveillance Surveillance Developmental Milestones and Normal Growth and immediate response to pathogens, tumor Tumor Inflammation cells, and foreign tissues and provide immunologic memory Memory Complex mental function having four distinct phases: (1) memorizing or learning, (2) retention, (3) recall, and (4) recognition. Clinically, it is usually subdivided into immediate, recent, and remote memory. Psychiatric Assessment.
Last updated: Dec 19, 2022
The initial process takes place in the outer cortex of the thymus Thymus A single, unpaired primary lymphoid organ situated in the mediastinum, extending superiorly into the neck to the lower edge of the thyroid gland and inferiorly to the fourth costal cartilage. It is necessary for normal development of immunologic function early in life. By puberty, it begins to involute and much of the tissue is replaced by fat. Lymphatic Drainage System: Anatomy, and the cells move into the deeper cortex as they mature.
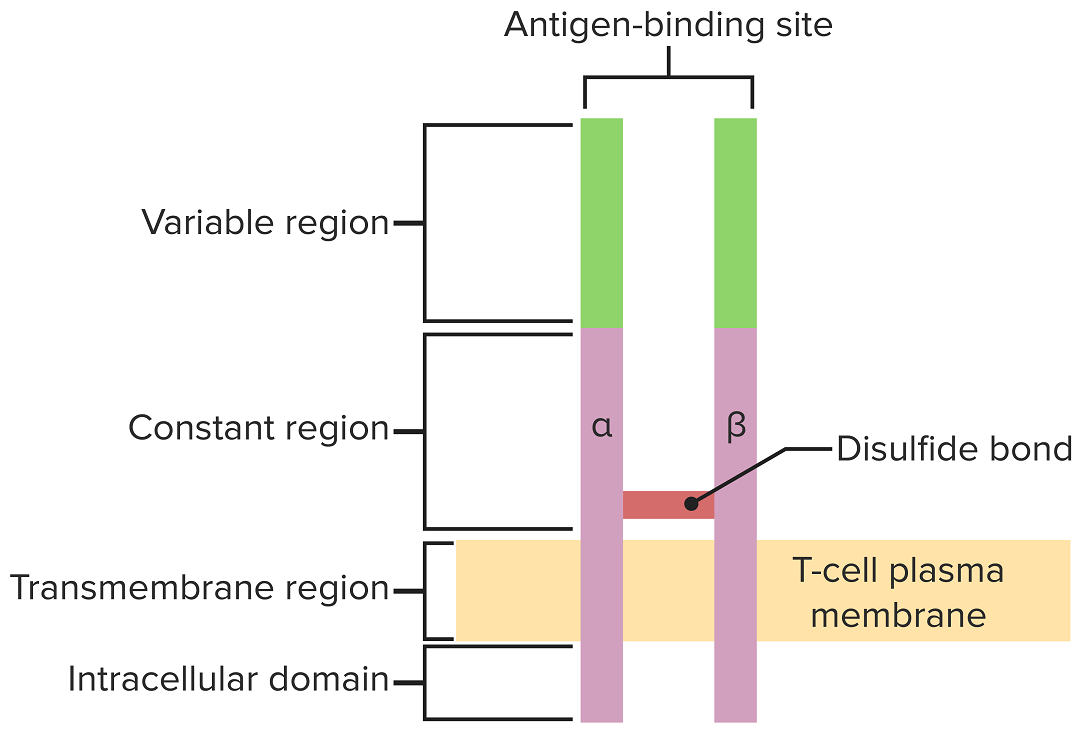
The T-cell receptor spans the cytoplasmic membrane and projects variable binding regions into the extracellular space to bind processed antigens associated with MHC I or MHC II molecules.
Image by Lecturio.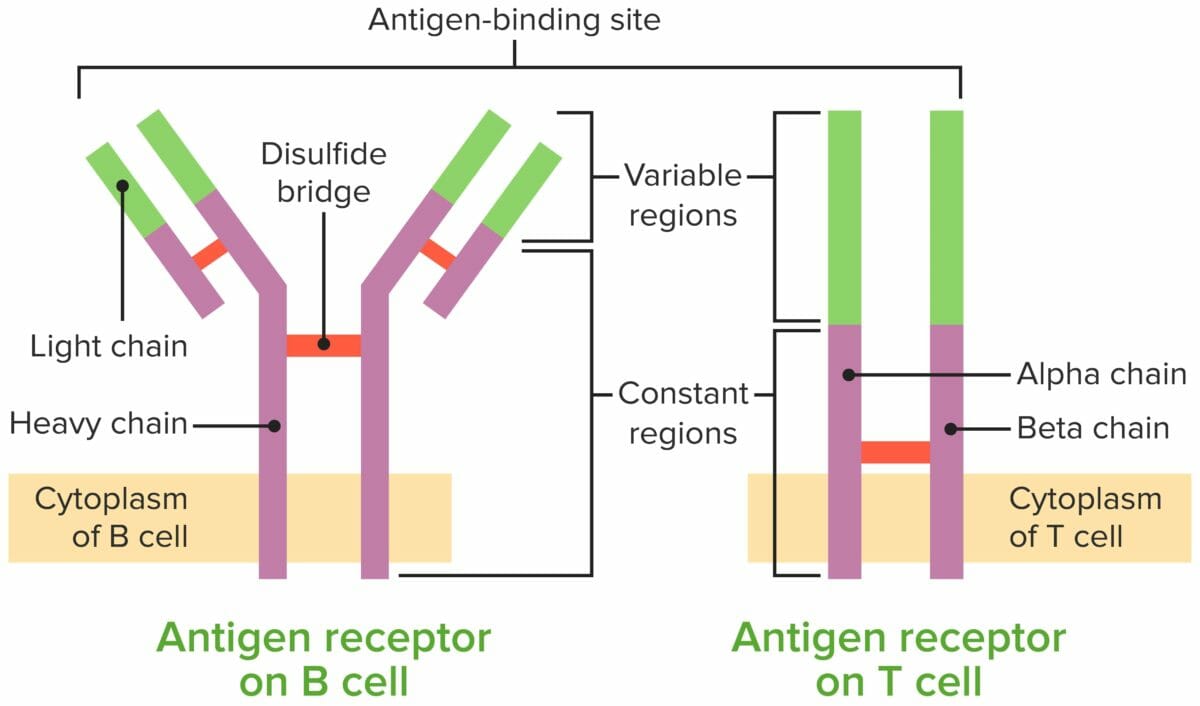
Comparison of the B-cell receptor (BCR) and the T-cell receptor (TCR)
Image by Lecturio.To reach functionality, the T cell goes through stages, released from the bone marrow Bone marrow The soft tissue filling the cavities of bones. Bone marrow exists in two types, yellow and red. Yellow marrow is found in the large cavities of large bones and consists mostly of fat cells and a few primitive blood cells. Red marrow is a hematopoietic tissue and is the site of production of erythrocytes and granular leukocytes. Bone marrow is made up of a framework of connective tissue containing branching fibers with the frame being filled with marrow cells. Bone Marrow: Composition and Hematopoiesis as progenitor cells to continue development in the thymus Thymus A single, unpaired primary lymphoid organ situated in the mediastinum, extending superiorly into the neck to the lower edge of the thyroid gland and inferiorly to the fourth costal cartilage. It is necessary for normal development of immunologic function early in life. By puberty, it begins to involute and much of the tissue is replaced by fat. Lymphatic Drainage System: Anatomy. The table summarizes the general steps taken.
| Maturation stage | T-cell receptor Receptor Receptors are proteins located either on the surface of or within a cell that can bind to signaling molecules known as ligands (e.g., hormones) and cause some type of response within the cell. Receptors | Associated events |
|---|---|---|
| Progenitor cell | None |
|
| DN cells | Rearrangement of β chain (pre-TCR): failure to rearrange leads to apoptosis Apoptosis A regulated cell death mechanism characterized by distinctive morphologic changes in the nucleus and cytoplasm, including the endonucleolytic cleavage of genomic DNA, at regularly spaced, internucleosomal sites, I.e., DNA fragmentation. It is genetically-programmed and serves as a balance to mitosis in regulating the size of animal tissues and in mediating pathologic processes associated with tumor growth. Ischemic Cell Damage |
|
| DP cells | Rearrangement of ɑ chain → ɑ chains assemble with β chains → complete ɑ–β TCR TCR Molecules on the surface of T-lymphocytes that recognize and combine with antigens. The receptors are non-covalently associated with a complex of several polypeptides collectively called CD3 antigens. Recognition of foreign antigen and the major histocompatibility complex is accomplished by a single heterodimeric antigen-receptor structure, composed of either alpha-beta or gamma-delta chains. Adaptive Cell-mediated Immunity–CD3 receptor Receptor Receptors are proteins located either on the surface of or within a cell that can bind to signaling molecules known as ligands (e.g., hormones) and cause some type of response within the cell. Receptors complex (expressed on the surface) |
|
| Single positive T cells |
|
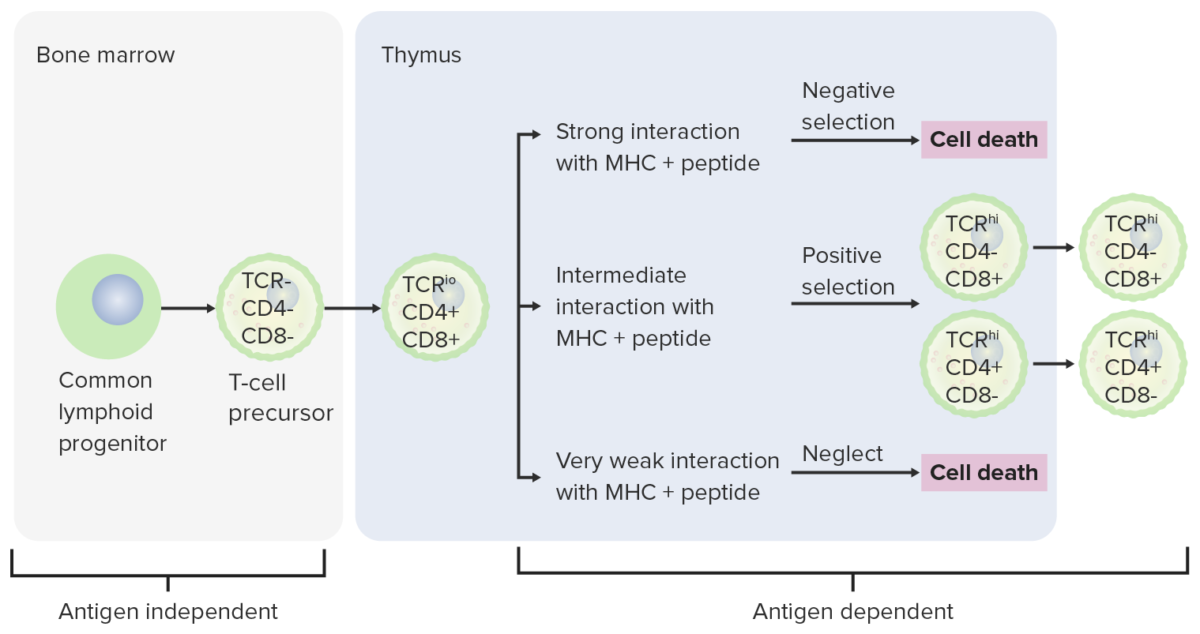
Differentiation stages of T cell:
From the bone marrow, progenitor cells go to the thymus for further maturation. The DN cells (no expression of CD4/CD8 or CD4–/CD8–) have not developed the TCR. The DN cells undergo rearrangement of the TCR gene and become pro-T cells, then pre-T cells. Through the series, CD4 and CD8 are expressed, and the TCR becomes assembled through gene rearrangements (DP cells). The thymus then presents MHC molecules to the developing T cells. Some cells undergo positive selection (intermediate interaction between MHC and TCR takes place) and produce functional cells. Some cells undergo negative selection (strong interaction between MHC and TCR), which results in cell death. The release of dysfunctional T cells, which can activate autoimmunity, is prevented. Some T cells fail to interact, leading to apoptosis. Mature T cells express either CD4 (T helper cells) or CD8 (cytotoxic T cells), not both.
Remember the “rule of 8”:
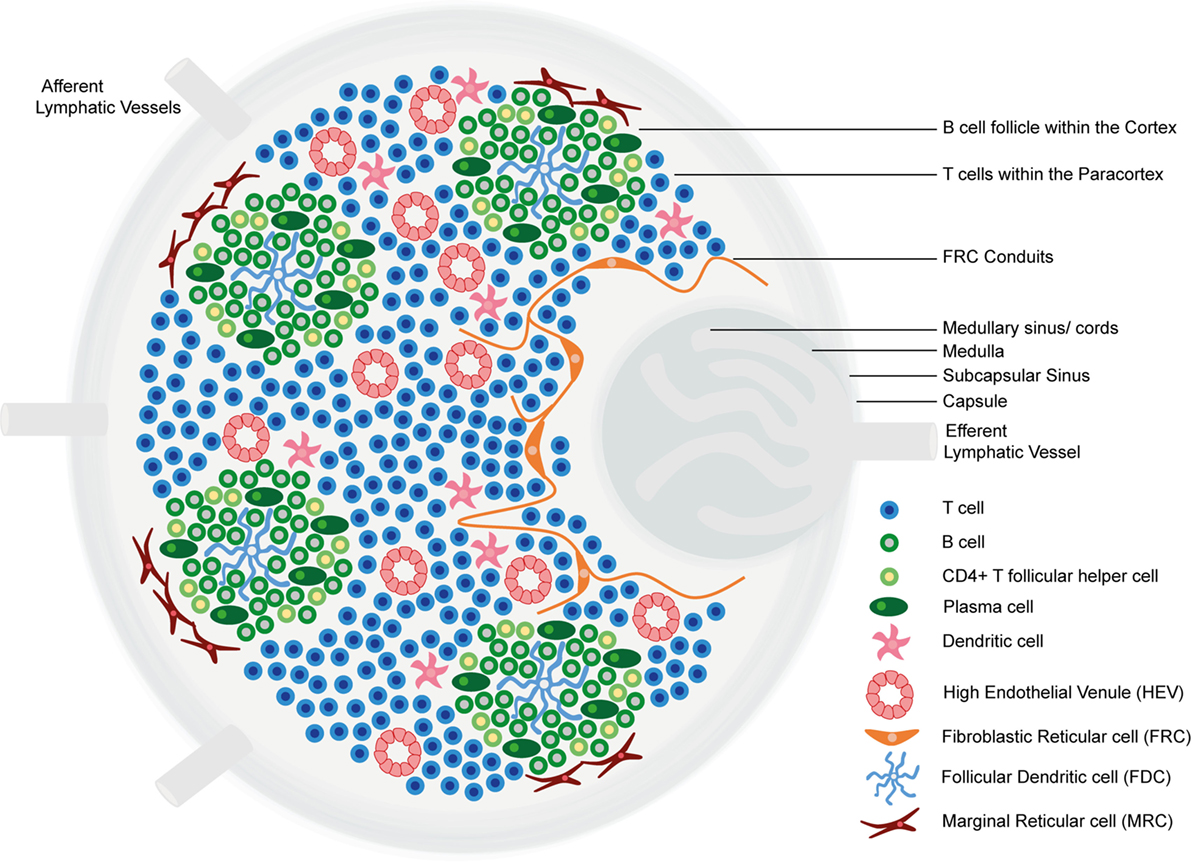
Structure and functional regions of a lymph node comprising a collagen-rich fibrous capsule and an underlying subcapsular sinus.
Cells are segregated into (1) the cortex (consisting of B cells, T follicular helper cells, and follicular dendritic cells arranged in primary follicles, in which B cells survey antigens presented on the follicular dendritic cell stromal network); and (2) the paracortex (accommodates T cells, dendritic cells, and fibroblastic reticular cells, which form stromal cell networks and reticular fibers).
The inner medulla is composed of lymphatic tissues (medullary cords) separated by medullary sinuses consisting of lymph.
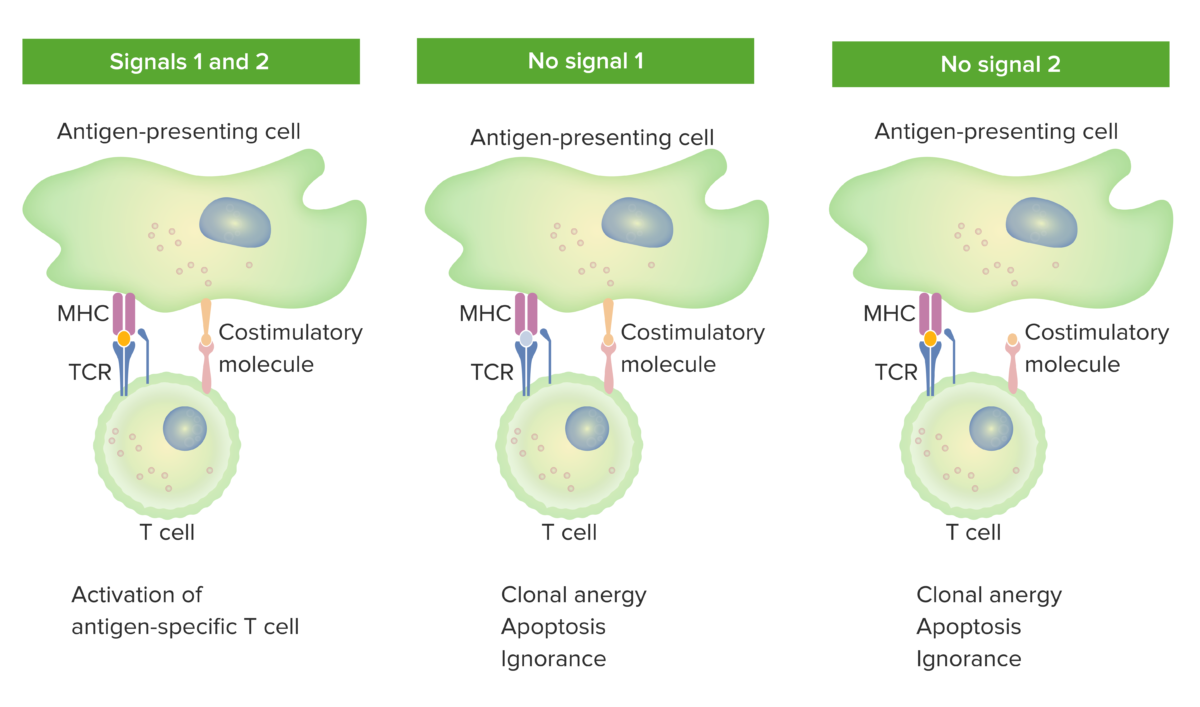
2-signal model of T-cell dependence on costimulation:
When both signal 1 (TCR binding the cognate antigen presented by the MHC molecule in the antigen-presenting cell) and signal 2 (costimulatory molecule interaction between the antigen-presenting cell and the T cell) are present, the mature T cell is fully activated.
The orange spot in the left panel indicates proper binding between antigen and TCR. However, when either signal 1 (middle image shows no antigen and TCR binding) or signal 2 (right image shows no costimulation) is missing, the T cell will not be fully activated.
Outcomes would be anergy (state of unresponsiveness), apoptosis (cell death), or ignorance (T cell does not notice or does not get affected by the antigen).
TCR: T-cell receptor
T helper cells have different cytokine profiles and roles in the immune response.
| CD4+ T cells | Differentiation stimulated by | Functions | Cytokines Cytokines Non-antibody proteins secreted by inflammatory leukocytes and some non-leukocytic cells, that act as intercellular mediators. They differ from classical hormones in that they are produced by a number of tissue or cell types rather than by specialized glands. They generally act locally in a paracrine or autocrine rather than endocrine manner. Adaptive Immune Response produced |
|---|---|---|---|
| Th1 |
|
|
|
| Th2 |
|
|
|
| Th17 |
|
Promote neutrophilic inflammation Inflammation Inflammation is a complex set of responses to infection and injury involving leukocytes as the principal cellular mediators in the body’s defense against pathogenic organisms. Inflammation is also seen as a response to tissue injury in the process of wound healing. The 5 cardinal signs of inflammation are pain, heat, redness, swelling, and loss of function. Inflammation |
|
| Tfh | IL-6 | Facilitate B-cell activation and maturation |
|
| Treg |
|
|
|
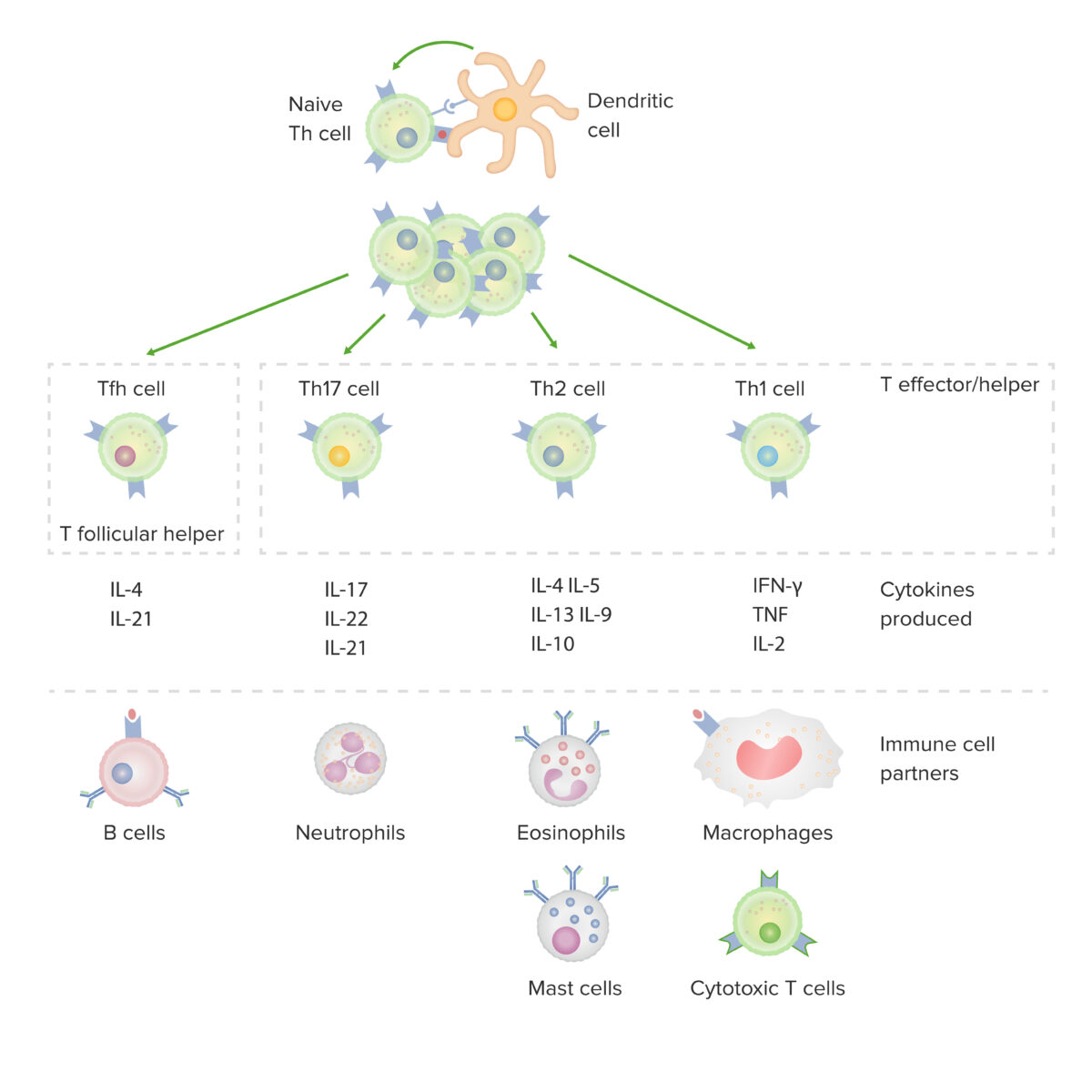
Subsets of CD4-positive helper T cells:
After activation by a dendritic cell, in the presence of particular cytokines, a naive CD4-positive T cell divides and differentiates into effector/helper (Th1, Th2 or Th17) or follicular helper (Tfh) subsets. Each type of cell produces cytokines that facilitate activation of other immune-cell partners.
IFN: interferon
TNF: tumor necrosis factor
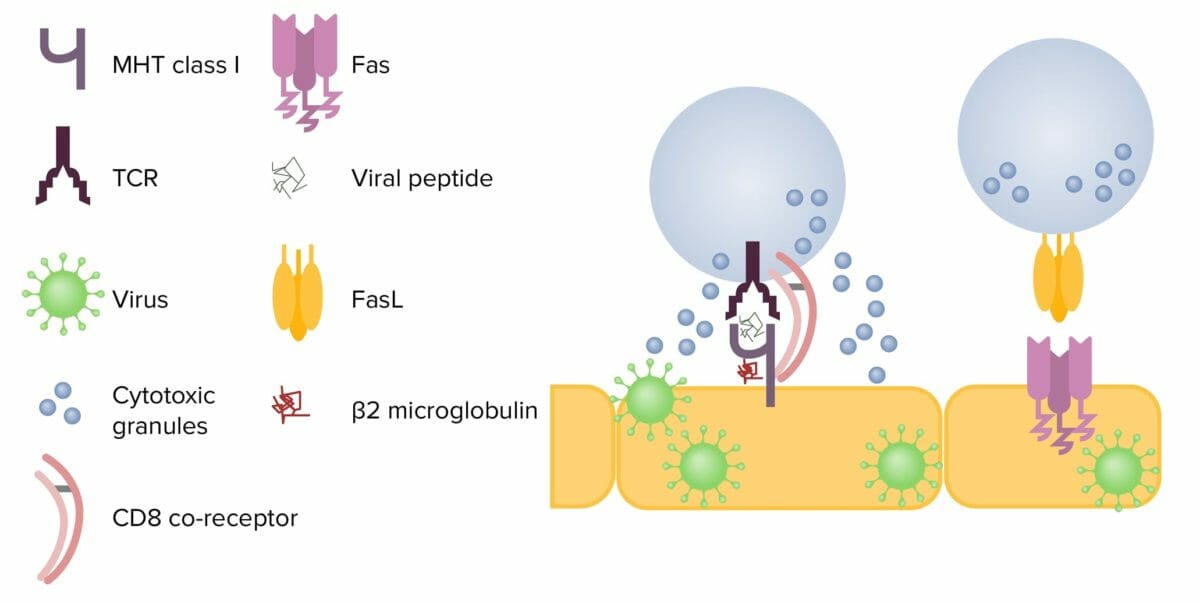
Mechanisms of cytotoxicity by CD8+ T cells:
Left: After contact with an infected cell, the T cell releases cytotoxic granules, perforin, and granzymes. Perforin creates a pore in the target cell membrane, allowing granzymes to enter the cell. These cleave proteins within the cell, ultimately resulting in apoptosis.
Right: When FasL interacts with Fas on a target cell, the caspase cascade is activated and results in apoptosis.
FasL: Fas ligand
TCR: T cell receptor
MHC: major histocompatibility complex
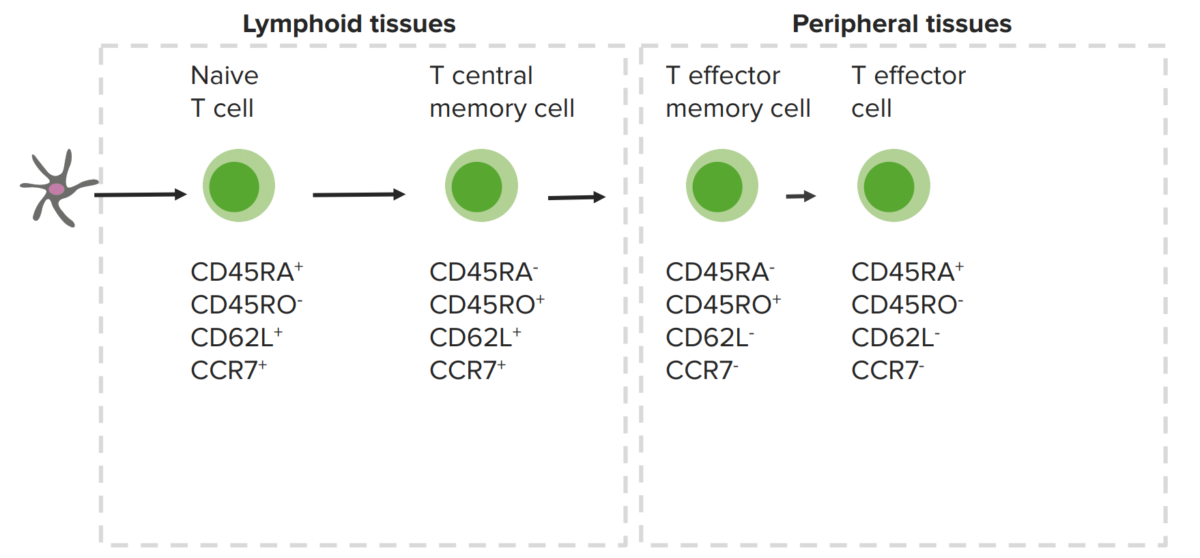
Memory T cells and expressed cellular markers:
The central memory T cells are in the lymphoid organs, while the peripheral memory T cells are in peripheral tissues.