B lymphocytes Lymphocytes Lymphocytes are heterogeneous WBCs involved in immune response. Lymphocytes develop from the bone marrow, starting from hematopoietic stem cells (HSCs) and progressing to common lymphoid progenitors (CLPs). B and T lymphocytes and natural killer (NK) cells arise from the lineage. Lymphocytes: Histology, also known as B cells, are important components of the adaptive immune system Immune system The body's defense mechanism against foreign organisms or substances and deviant native cells. It includes the humoral immune response and the cell-mediated response and consists of a complex of interrelated cellular, molecular, and genetic components. Primary Lymphatic Organs. In the bone marrow Bone marrow The soft tissue filling the cavities of bones. Bone marrow exists in two types, yellow and red. Yellow marrow is found in the large cavities of large bones and consists mostly of fat cells and a few primitive blood cells. Red marrow is a hematopoietic tissue and is the site of production of erythrocytes and granular leukocytes. Bone marrow is made up of a framework of connective tissue containing branching fibers with the frame being filled with marrow cells. Bone Marrow: Composition and Hematopoiesis, the hematopoietic stem cells Hematopoietic stem cells Progenitor cells from which all blood cells derived. They are found primarily in the bone marrow and also in small numbers in the peripheral blood. Bone Marrow: Composition and Hematopoiesis go through a series of steps to become mature naive B cells. The cells migrate to secondary lymphoid organs Lymphoid organs A system of organs and tissues that process and transport immune cells and lymph. Primary Lymphatic Organs for activation and further maturation. The process entails antigen Antigen Substances that are recognized by the immune system and induce an immune reaction. Vaccination stimulation, with or without the help of T cells T cells Lymphocytes responsible for cell-mediated immunity. Two types have been identified - cytotoxic (t-lymphocytes, cytotoxic) and helper T-lymphocytes (t-lymphocytes, helper-inducer). They are formed when lymphocytes circulate through the thymus gland and differentiate to thymocytes. When exposed to an antigen, they divide rapidly and produce large numbers of new T cells sensitized to that antigen. T cells: Types and Functions. The T-cell–independent activation generates a short-lived immune response (via plasma Plasma The residual portion of blood that is left after removal of blood cells by centrifugation without prior blood coagulation. Transfusion Products cells), and this is seen with antigens such as bacterial lipopolysaccharides Lipopolysaccharides Lipid-containing polysaccharides which are endotoxins and important group-specific antigens. They are often derived from the cell wall of gram-negative bacteria and induce immunoglobulin secretion. The lipopolysaccharide molecule consists of three parts: lipid a, core polysaccharide, and o-specific chains (o antigens). When derived from Escherichia coli, lipopolysaccharides serve as polyclonal b-cell mitogens commonly used in laboratory immunology. Diarrheagenic E. coli. T-cell–dependent activation, on the other hand Hand The hand constitutes the distal part of the upper limb and provides the fine, precise movements needed in activities of daily living. It consists of 5 metacarpal bones and 14 phalanges, as well as numerous muscles innervated by the median and ulnar nerves. Hand: Anatomy, produces both plasma Plasma The residual portion of blood that is left after removal of blood cells by centrifugation without prior blood coagulation. Transfusion Products cells and memory Memory Complex mental function having four distinct phases: (1) memorizing or learning, (2) retention, (3) recall, and (4) recognition. Clinically, it is usually subdivided into immediate, recent, and remote memory. Psychiatric Assessment cells. Activated B cells Activated B cells Humoral Adaptive Immunity then proliferate in the germinal centers, but not all become effector B cells. Through somatic hypermutation, B cells undergo additional mechanisms to increase the affinity of the antibody to the antigen Antigen Substances that are recognized by the immune system and induce an immune reaction. Vaccination. Only those with high-affinity B-cell receptors Receptors Receptors are proteins located either on the surface of or within a cell that can bind to signaling molecules known as ligands (e.g., hormones) and cause some type of response within the cell. Receptors subsequently advance for terminal differentiation. B cells then go through class switching (from IgM IgM A class of immunoglobulin bearing mu chains (immunoglobulin mu-chains). Igm can fix complement. The name comes from its high molecular weight and originally being called a macroglobulin. Immunoglobulins: Types and Functions to another class of Ig Ig X-linked Agammaglobulinemia) under the influence of cytokines Cytokines Non-antibody proteins secreted by inflammatory leukocytes and some non-leukocytic cells, that act as intercellular mediators. They differ from classical hormones in that they are produced by a number of tissue or cell types rather than by specialized glands. They generally act locally in a paracrine or autocrine rather than endocrine manner. Adaptive Immune Response. After class switching, the B cells become plasma Plasma The residual portion of blood that is left after removal of blood cells by centrifugation without prior blood coagulation. Transfusion Products cells (which produce antibodies Antibodies Immunoglobulins (Igs), also known as antibodies, are glycoprotein molecules produced by plasma cells that act in immune responses by recognizing and binding particular antigens. The various Ig classes are IgG (the most abundant), IgM, IgE, IgD, and IgA, which differ in their biologic features, structure, target specificity, and distribution. Immunoglobulins: Types and Functions) or memory Memory Complex mental function having four distinct phases: (1) memorizing or learning, (2) retention, (3) recall, and (4) recognition. Clinically, it is usually subdivided into immediate, recent, and remote memory. Psychiatric Assessment cells (which mount a robust secondary immune response Secondary immune response Humoral Adaptive Immunity).
Last updated: Dec 19, 2022
B (bursa-derived) lymphocytes Lymphocytes Lymphocytes are heterogeneous WBCs involved in immune response. Lymphocytes develop from the bone marrow, starting from hematopoietic stem cells (HSCs) and progressing to common lymphoid progenitors (CLPs). B and T lymphocytes and natural killer (NK) cells arise from the lineage. Lymphocytes: Histology, or B cells, are a type of lymphocyte that arises from the common lymphoid progenitor.
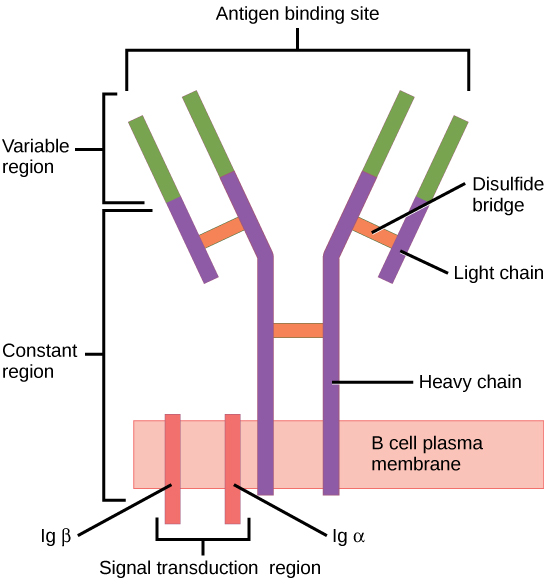
The B-cell receptor (BCR) consists of the Ig molecule and the signaling molecule:
Ig contains 2 identical heavy chains and 2 identical light chains linked by a disulfide bridge. The membrane-bound Ig is anchored to the cell surface.
To reach functionality, the B cell goes through stages in the bone marrow Bone marrow The soft tissue filling the cavities of bones. Bone marrow exists in two types, yellow and red. Yellow marrow is found in the large cavities of large bones and consists mostly of fat cells and a few primitive blood cells. Red marrow is a hematopoietic tissue and is the site of production of erythrocytes and granular leukocytes. Bone marrow is made up of a framework of connective tissue containing branching fibers with the frame being filled with marrow cells. Bone Marrow: Composition and Hematopoiesis and the secondary lymphoid organs Lymphoid organs A system of organs and tissues that process and transport immune cells and lymph. Primary Lymphatic Organs:
| Maturation stage | Ig Ig X-linked Agammaglobulinemia genes Genes A category of nucleic acid sequences that function as units of heredity and which code for the basic instructions for the development, reproduction, and maintenance of organisms. DNA Types and Structure | B-cell receptor Receptor Receptors are proteins located either on the surface of or within a cell that can bind to signaling molecules known as ligands (e.g., hormones) and cause some type of response within the cell. Receptors ( BCR BCR Lymphocytes: Histology) | Associated events |
|---|---|---|---|
| Antigen-independent | |||
| Pre-pro-B cell Pre-pro-B cell Lymphocytes: Histology | Germ-line DNA DNA A deoxyribonucleotide polymer that is the primary genetic material of all cells. Eukaryotic and prokaryotic organisms normally contain DNA in a double-stranded state, yet several important biological processes transiently involve single-stranded regions. DNA, which consists of a polysugar-phosphate backbone possessing projections of purines (adenine and guanine) and pyrimidines (thymine and cytosine), forms a double helix that is held together by hydrogen bonds between these purines and pyrimidines (adenine to thymine and guanine to cytosine). DNA Types and Structure | None | No heavy- or light-chain expression |
| Pro-B cell Pro-B cell Lymphocytes: Histology | IGH D-J rearranged | None | Starts to express CD19, CD34, and HLA-DR (class II histocompatibility antigen Antigen Substances that are recognized by the immune system and induce an immune reaction. Vaccination) |
| Pre-B cell | IGH V-D-J rearranged | Pre-BCR is formed:
|
Other markers appear (CD79, CD10, CD20, CD40 CD40 Members of the tumor necrosis factor receptor superfamily with specificity for CD40 ligand. They are found on mature B-lymphocytes, some epithelial cells; and lymphoid dendritic cells. Evidence suggests that CD40-dependent activation of B-cells is important for generation of memory B-cells within the germinal centers. Mutations in the CD40 antigen gene result in hyper-igm immunodeficiency syndrome, type 3. Signaling of the receptor occurs through its association with tnf receptor-associated factors. Hyper-IgM Syndrome, and terminal deoxynucleotidyl transferase Terminal deoxynucleotidyl transferase A non-template-directed DNA polymerase normally found in vertebrate thymus and bone marrow. It catalyzes the elongation of oligo- or polydeoxynucleotide chains and is widely used as a tool in the differential diagnosis of acute leukemias in man. Acute Lymphoblastic Leukemia among them). |
| Immature B cell Immature B cell Lymphocytes: Histology |
|
Mature BCR BCR Lymphocytes: Histology ( IgM IgM A class of immunoglobulin bearing mu chains (immunoglobulin mu-chains). Igm can fix complement. The name comes from its high molecular weight and originally being called a macroglobulin. Immunoglobulins: Types and Functions molecule) | HLA-DR, CD19, CD20, and CD40 CD40 Members of the tumor necrosis factor receptor superfamily with specificity for CD40 ligand. They are found on mature B-lymphocytes, some epithelial cells; and lymphoid dendritic cells. Evidence suggests that CD40-dependent activation of B-cells is important for generation of memory B-cells within the germinal centers. Mutations in the CD40 antigen gene result in hyper-igm immunodeficiency syndrome, type 3. Signaling of the receptor occurs through its association with tnf receptor-associated factors. Hyper-IgM Syndrome expression continues, but not the other markers (e.g., CD10, CD34, and terminal deoxynucleotidyl transferase Terminal deoxynucleotidyl transferase A non-template-directed DNA polymerase normally found in vertebrate thymus and bone marrow. It catalyzes the elongation of oligo- or polydeoxynucleotide chains and is widely used as a tool in the differential diagnosis of acute leukemias in man. Acute Lymphoblastic Leukemia). |
| Mature B cell Mature B cell Lymphocytes: Histology (naive) |
|
With mature BCR BCR Lymphocytes: Histology ( IgM IgM A class of immunoglobulin bearing mu chains (immunoglobulin mu-chains). Igm can fix complement. The name comes from its high molecular weight and originally being called a macroglobulin. Immunoglobulins: Types and Functions) → exit bone marrow Bone marrow The soft tissue filling the cavities of bones. Bone marrow exists in two types, yellow and red. Yellow marrow is found in the large cavities of large bones and consists mostly of fat cells and a few primitive blood cells. Red marrow is a hematopoietic tissue and is the site of production of erythrocytes and granular leukocytes. Bone marrow is made up of a framework of connective tissue containing branching fibers with the frame being filled with marrow cells. Bone Marrow: Composition and Hematopoiesis | All express CD19 and CD20. |
| Antigen-dependent | |||
| Mature B cell Mature B cell Lymphocytes: Histology (in secondary lymphoid tissues) | Mature BCR BCR Lymphocytes: Histology (expresses IgM IgM A class of immunoglobulin bearing mu chains (immunoglobulin mu-chains). Igm can fix complement. The name comes from its high molecular weight and originally being called a macroglobulin. Immunoglobulins: Types and Functions and IgD IgD An immunoglobulin which accounts for less than 1% of plasma immunoglobulin. It is found on the membrane of many circulating B lymphocytes. Immunoglobulins: Types and Functions once within the secondary lymphoid tissues) | Cells can rest or B-cell activation can occur: B cells interact with exogenous antigen Antigen Substances that are recognized by the immune system and induce an immune reaction. Vaccination and/or T helper cells. | |
| Activated B cell Activated B cell Lymphocytes: Histology | Class switching | Once activated, can switch to IgE IgE An immunoglobulin associated with mast cells. Overexpression has been associated with allergic hypersensitivity. Immunoglobulins: Types and Functions, IgG IgG The major immunoglobulin isotype class in normal human serum. There are several isotype subclasses of igg, for example, igg1, igg2a, and igg2b. Hypersensitivity Pneumonitis, IgA IgA Represents 15-20% of the human serum immunoglobulins, mostly as the 4-chain polymer in humans or dimer in other mammals. Secretory iga is the main immunoglobulin in secretions. Immunoglobulins: Types and Functions, or remain as IgM IgM A class of immunoglobulin bearing mu chains (immunoglobulin mu-chains). Igm can fix complement. The name comes from its high molecular weight and originally being called a macroglobulin. Immunoglobulins: Types and Functions | |
| Memory Memory Complex mental function having four distinct phases: (1) memorizing or learning, (2) retention, (3) recall, and (4) recognition. Clinically, it is usually subdivided into immediate, recent, and remote memory. Psychiatric Assessment B cell |
|
||
| Plasma Plasma The residual portion of blood that is left after removal of blood cells by centrifugation without prior blood coagulation. Transfusion Products cell |
|
||
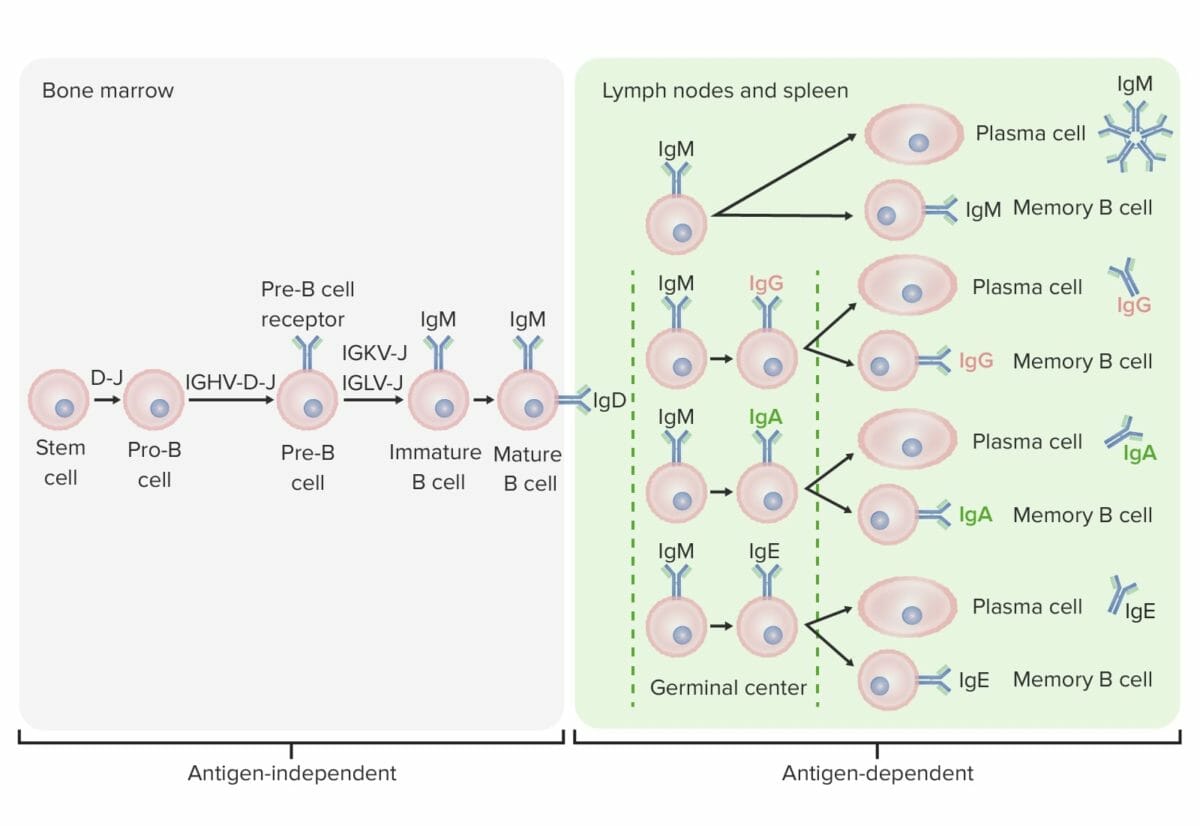
Differentiation stages of the B cell:
In antigen-independent stages, B-cell production starts with the hematopoietic stem cell (HSC), which becomes a common lymphoid progenitor (CLP) and then a pre-pro-B cell or B-progenitor cell. The next steps include gene rearrangement to assemble the Ig molecule. Ig heavy chains start with rearrangement of diversity and joining segments to form the pro-B cell. In the next step (pre-B cell), Ig heavy-chain recombination (variable, diversity, joining) is completed and the pre-B-cell receptor is formed. Light-chain (kappa (κ) or lambda (λ)) rearrangement occurs, resulting in the expression of a complete IgM-antibody molecule by an immature B cell. Formation of the mature B cell (naive) with both IgM and IgD follows.
Antigen-dependent stages take place in secondary lymphoid tissues. Once the mature B cell produce IgM and IgD, a class switch can take place to make IgE, IgG, and IgA. B cells are activated and become plasma cells or memory cells.
The B cell migrates from the bone marrow Bone marrow The soft tissue filling the cavities of bones. Bone marrow exists in two types, yellow and red. Yellow marrow is found in the large cavities of large bones and consists mostly of fat cells and a few primitive blood cells. Red marrow is a hematopoietic tissue and is the site of production of erythrocytes and granular leukocytes. Bone marrow is made up of a framework of connective tissue containing branching fibers with the frame being filled with marrow cells. Bone Marrow: Composition and Hematopoiesis to the secondary lymphoid organs Lymphoid organs A system of organs and tissues that process and transport immune cells and lymph. Primary Lymphatic Organs. This process takes a number of measures to produce a functional differentiated B cell: activation by an antigen Antigen Substances that are recognized by the immune system and induce an immune reaction. Vaccination, proliferation, affinity maturation Affinity maturation Humoral Adaptive Immunity, class switching, and differentiation (into plasma Plasma The residual portion of blood that is left after removal of blood cells by centrifugation without prior blood coagulation. Transfusion Products or memory Memory Complex mental function having four distinct phases: (1) memorizing or learning, (2) retention, (3) recall, and (4) recognition. Clinically, it is usually subdivided into immediate, recent, and remote memory. Psychiatric Assessment cell).
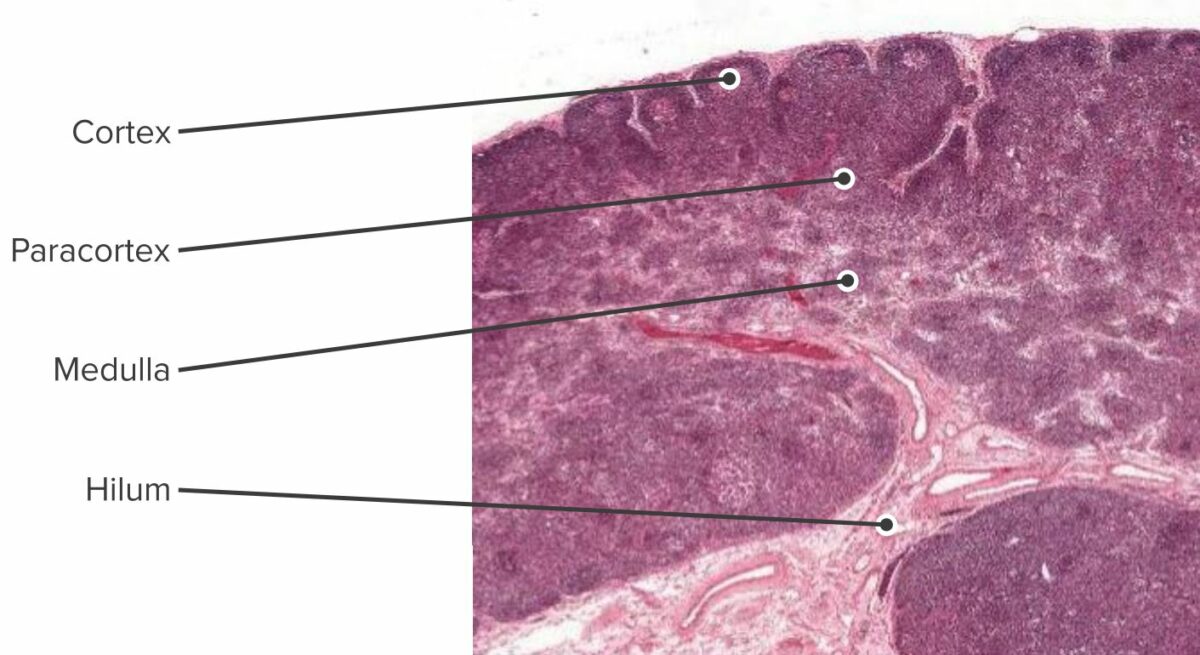
Histologic section of the lymph node showing the cortex, paracortex and the medulla
Image by Geoffrey Meyer, edited by Lecturio.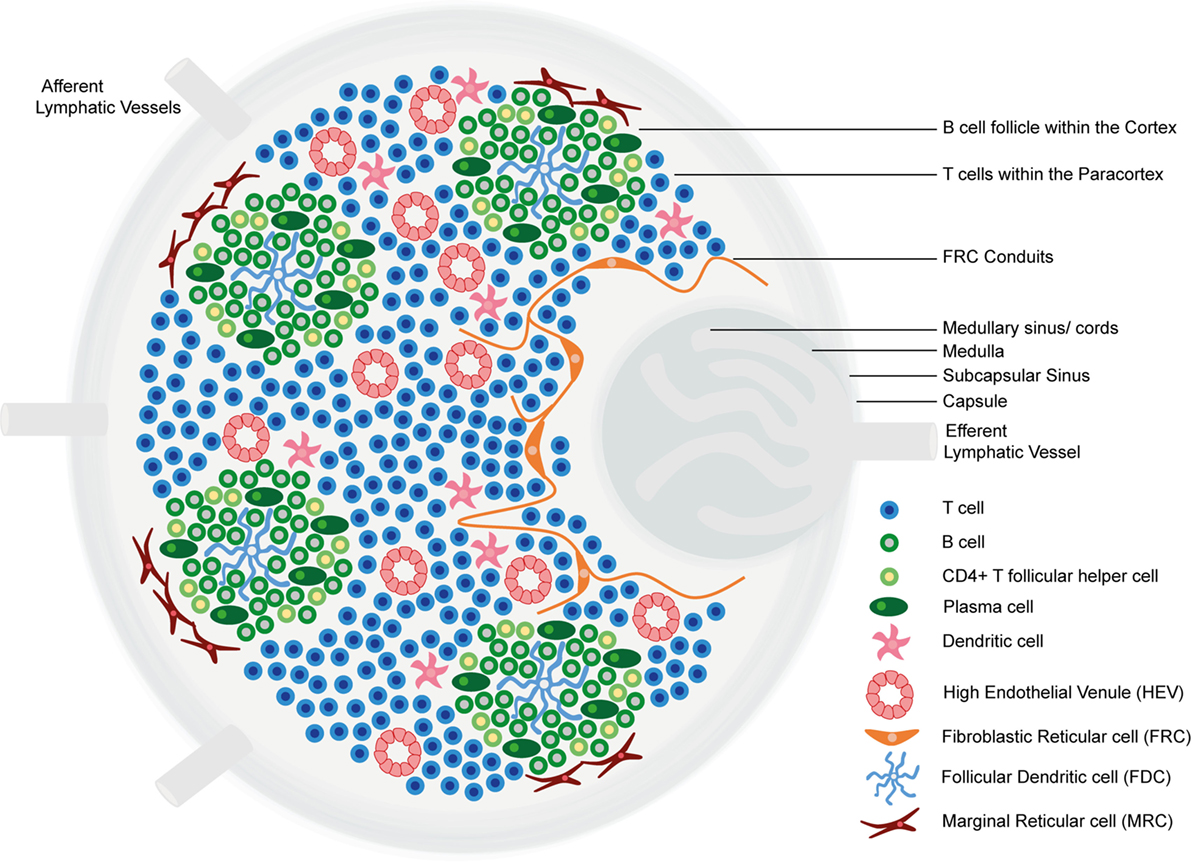
Structure and functional regions of a lymph node: comprise a collagen-rich fibrous capsule and an underlying subcapsular sinus (SCS).
Cells are segregated into (1) the cortex (consisting of B cells, T follicular helper cells, and follicular dendritic cells [FDCs] arranged in primary follicles, in which B cells survey antigens presented on the FDC stromal network); and (2) the paracortex (accommodates T cells, dendritic cells [DCs], and fibroblastic reticular cells [FRCs] which form stromal cell networks and reticular fibers).
The inner medulla is composed of lymphatic tissues (medullary cords) separated by medullary sinuses consisting of lymph.
B-cell activation by antigen Antigen Substances that are recognized by the immune system and induce an immune reaction. Vaccination presentation can have different paths:
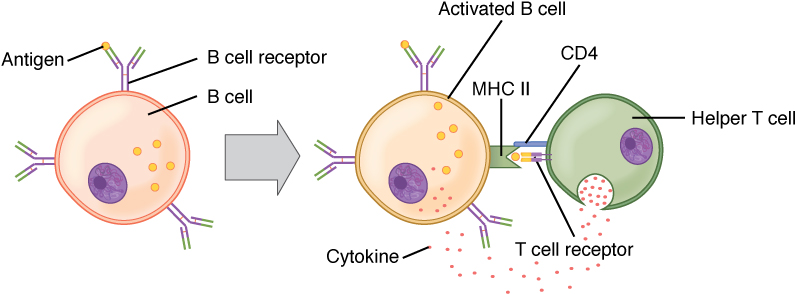
B-cell activation (T-cell-dependent):
Circulating antigen interacts with the BCR of the B cell. The antigen is endocytosed and degraded and the peptide components are complexed with cell surface MHC II molecules. T follicular helper (Tfh) cells (specialized CD4+ T helper cells) recognize and bind the antigen–MHC II complex. Cytokines are released by the Tfh cells, leading to B-cell activation and proliferation. Activated B cells enter the germinal centers, where they continue the process, leading to differentiation.
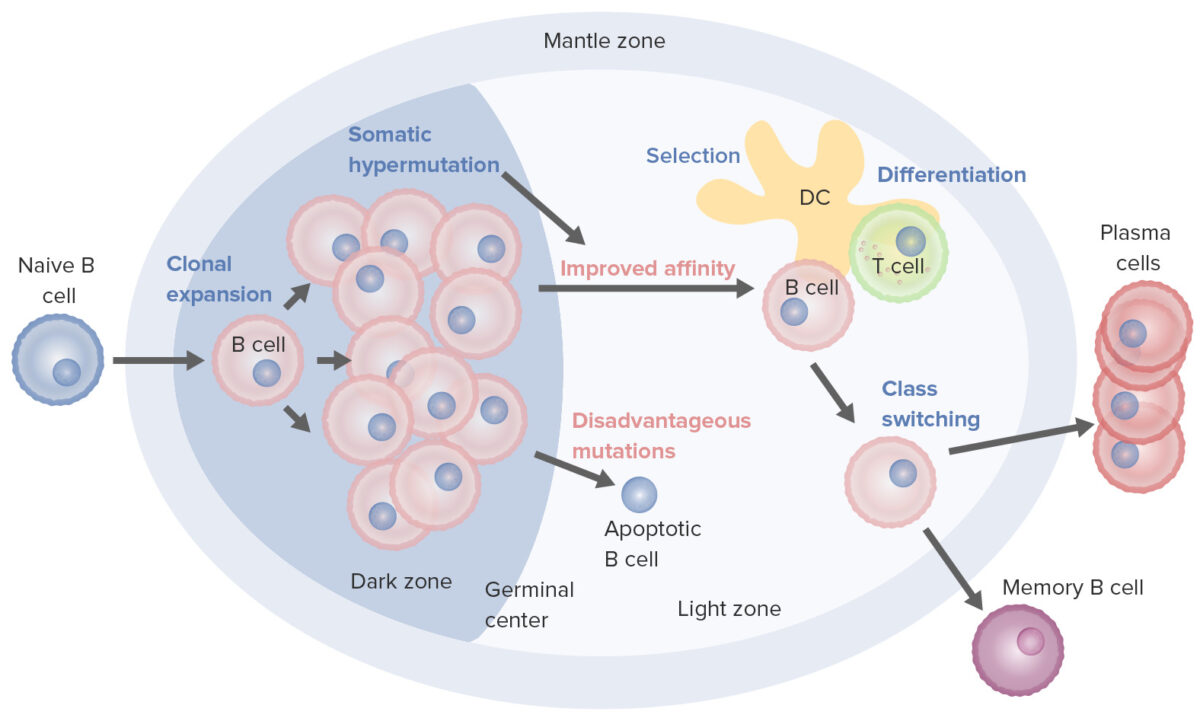
B-cell activation and maturation processes taking place in the germinal center:
On activation, the B cell moves from the mantle zone and enters the germinal center. B-cell proliferation (clonal expansion) takes place and antibody affinity to the antigen is enhanced through the process of somatic hypermutation. Repeated cycles of proliferation and hypermutation fine-tune the B-cell receptor. However, not all B cells continue to differentiate, especially if the affinity is weak. Apoptosis follows if the antigen–antibody binding is not optimized. Those with strong affinity survive (selection), with the help of survival signals from follicular dendritic cells (DC) and T cells. These selected B cells move on to class switching and differentiation into plasma cells or memory cells.
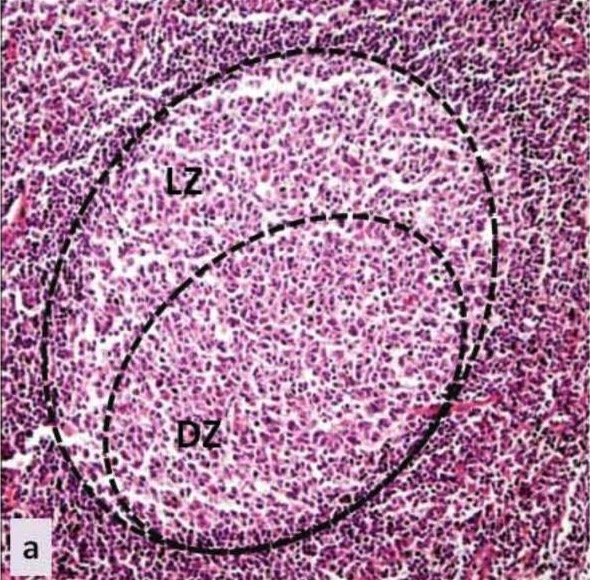
Germinal center: histology of the germinal center of a secondary lymphoid tissue
LZ: light zone
DZ: dark zone
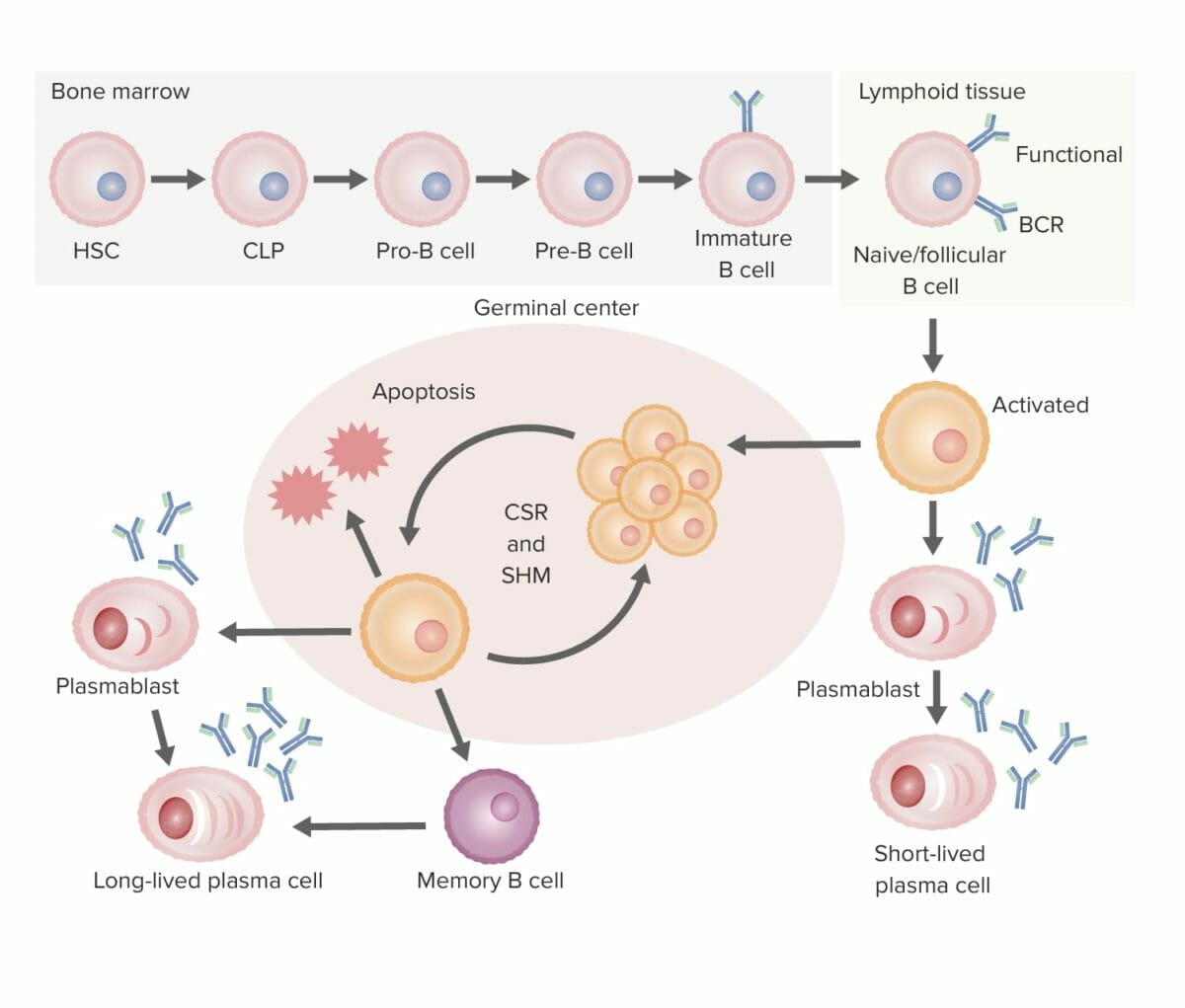
Summary of B-cell development to differentiation (from bone marrow to secondary lymphoid organ):
B-cell development:
In the bone marrow, B cells develop into immature B cells, a process in which the B-cell receptor (BCR) is assembled. Then the B cell migrates to the secondary lymphoid organs, where activation occurs.
B-cell activation:
The antigen binds the B cell with the “best match” BCR. One pathway of activation is T-cell–independent, whereby the activated B cell is triggered to differentiate into a short-lived plasma cell (producing antibodies) without the help of the T cell. In T-cell–dependent activation, the T cell recognizes the antigen–MHC II and triggers proliferation of the B cell in the germinal center of the lymphoid tissue.
Proliferation and maturation:
The process is followed by somatic hypermutation (SHM; a programmed mutation to further fine-tune the affinity of the antibody to the antigen). Repeated cycles of proliferation and hypermutation refine the BCR. Only those with the best affinity will be selected and survive; those with low affinity will undergo apoptosis. The surviving B cells then go through class-switch recombination (CSR), in which the heavy chain makeup is changed (IgM to other isotypes) with the help of cytokines.
Differentiation:
These B cells then differentiate into plasma cells and memory cells, leaving the germinal center.
From the initial B-cell production, many processes allow humans to produce different antibody molecules that are significantly more than the number of genes Genes A category of nucleic acid sequences that function as units of heredity and which code for the basic instructions for the development, reproduction, and maintenance of organisms. DNA Types and Structure in the genome Genome The complete genetic complement contained in the DNA of a set of chromosomes in a human. The length of the human genome is about 3 billion base pairs. Basic Terms of Genetics.
It is estimated that billions of antibodies Antibodies Immunoglobulins (Igs), also known as antibodies, are glycoprotein molecules produced by plasma cells that act in immune responses by recognizing and binding particular antigens. The various Ig classes are IgG (the most abundant), IgM, IgE, IgD, and IgA, which differ in their biologic features, structure, target specificity, and distribution. Immunoglobulins: Types and Functions are generated, compared to about 30,000 genes Genes A category of nucleic acid sequences that function as units of heredity and which code for the basic instructions for the development, reproduction, and maintenance of organisms. DNA Types and Structure.
The immune system Immune system The body’s defense mechanism against foreign organisms or substances and deviant native cells. It includes the humoral immune response and the cell-mediated response and consists of a complex of interrelated cellular, molecular, and genetic components. Primary Lymphatic Organs has unique mechanisms to create antibody diversity, which include: