Antimycobacterial agents represent a diverse group of compounds that have activity against mycobacterial infections Infections Invasion of the host organism by microorganisms or their toxins or by parasites that can cause pathological conditions or diseases. Chronic Granulomatous Disease, including tuberculosis Tuberculosis Tuberculosis (TB) is an infectious disease caused by Mycobacterium tuberculosis complex bacteria. The bacteria usually attack the lungs but can also damage other parts of the body. Approximately 30% of people around the world are infected with this pathogen, with the majority harboring a latent infection. Tuberculosis spreads through the air when a person with active pulmonary infection coughs or sneezes. Tuberculosis, leprosy Leprosy Leprosy, also known as Hansen's disease, is a chronic bacterial infection caused by Mycobacterium leprae complex bacteria. Symptoms primarily affect the skin and peripheral nerves, resulting in cutaneous manifestations (e.g., hypopigmented macules) and neurologic manifestations (e.g., loss of sensation). Leprosy and Mycobacterium avium Mycobacterium avium A bacterium causing tuberculosis in domestic fowl and other birds. In pigs, it may cause localized and sometimes disseminated disease. The organism occurs occasionally in sheep and cattle. It should be distinguished from the m. avium complex, which infects primarily humans. Mycobacterium complex (MAC) disease. The 1st-line agents for tuberculosis Tuberculosis Tuberculosis (TB) is an infectious disease caused by Mycobacterium tuberculosis complex bacteria. The bacteria usually attack the lungs but can also damage other parts of the body. Approximately 30% of people around the world are infected with this pathogen, with the majority harboring a latent infection. Tuberculosis spreads through the air when a person with active pulmonary infection coughs or sneezes. Tuberculosis are rifampin Rifampin A semisynthetic antibiotic produced from streptomyces mediterranei. It has a broad antibacterial spectrum, including activity against several forms of Mycobacterium. In susceptible organisms it inhibits dna-dependent RNA polymerase activity by forming a stable complex with the enzyme. It thus suppresses the initiation of RNA synthesis. Rifampin is bactericidal, and acts on both intracellular and extracellular organisms. Epiglottitis, isoniazid, pyrazinamide, and ethambutol. The drugs vary in their mechanisms of action: rifampin Rifampin A semisynthetic antibiotic produced from streptomyces mediterranei. It has a broad antibacterial spectrum, including activity against several forms of Mycobacterium. In susceptible organisms it inhibits dna-dependent RNA polymerase activity by forming a stable complex with the enzyme. It thus suppresses the initiation of RNA synthesis. Rifampin is bactericidal, and acts on both intracellular and extracellular organisms. Epiglottitis inhibits RNA RNA A polynucleotide consisting essentially of chains with a repeating backbone of phosphate and ribose units to which nitrogenous bases are attached. RNA is unique among biological macromolecules in that it can encode genetic information, serve as an abundant structural component of cells, and also possesses catalytic activity. RNA Types and Structure synthesis Synthesis Polymerase Chain Reaction (PCR), isoniazid inhibits mycolic acid Mycolic acid Long fatty acids found in the cell walls of some actinobacteria, including Mycobacterium tuberculosis. Tuberculosis synthesis Synthesis Polymerase Chain Reaction (PCR), pyrazinamide acts on membrane transport and protein synthesis Synthesis Polymerase Chain Reaction (PCR), and ethambutol prevents cell wall Cell wall The outermost layer of a cell in most plants; bacteria; fungi; and algae. The cell wall is usually a rigid structure that lies external to the cell membrane, and provides a protective barrier against physical or chemical agents. Cell Types: Eukaryotic versus Prokaryotic synthesis Synthesis Polymerase Chain Reaction (PCR). Monotherapy is not recommended because of the increased risk of drug resistance Resistance Physiologically, the opposition to flow of air caused by the forces of friction. As a part of pulmonary function testing, it is the ratio of driving pressure to the rate of air flow. Ventilation: Mechanics of Breathing. Multidrug treatment takes several months and requires sputum monitoring. As for leprosy Leprosy Leprosy, also known as Hansen's disease, is a chronic bacterial infection caused by Mycobacterium leprae complex bacteria. Symptoms primarily affect the skin and peripheral nerves, resulting in cutaneous manifestations (e.g., hypopigmented macules) and neurologic manifestations (e.g., loss of sensation). Leprosy, an infection due to Mycobacterium leprae Mycobacterium leprae A species of gram-positive, aerobic bacteria that causes leprosy in man. Its organisms are generally arranged in clumps, rounded masses, or in groups of bacilli side by side. Mycobacterium, rifampin Rifampin A semisynthetic antibiotic produced from streptomyces mediterranei. It has a broad antibacterial spectrum, including activity against several forms of Mycobacterium. In susceptible organisms it inhibits dna-dependent RNA polymerase activity by forming a stable complex with the enzyme. It thus suppresses the initiation of RNA synthesis. Rifampin is bactericidal, and acts on both intracellular and extracellular organisms. Epiglottitis is also used, with dapsone. The lepromatous form Lepromatous form A chronic communicable infection which is a principal or polar form of leprosy. This disorder is caused by Mycobacterium leprae and produces diffuse granulomatous skin lesions in the form of nodules, macules, or papules. The peripheral nerves are involved symmetrically and neural sequelae occur in the advanced stage. Leprosy requires a 3rd agent (clofazimine). Pulmonary infections Infections Invasion of the host organism by microorganisms or their toxins or by parasites that can cause pathological conditions or diseases. Chronic Granulomatous Disease with MAC are managed with macrolides Macrolides Macrolides and ketolides are antibiotics that inhibit bacterial protein synthesis by binding to the 50S ribosomal subunit and blocking transpeptidation. These antibiotics have a broad spectrum of antimicrobial activity but are best known for their coverage of atypical microorganisms. Macrolides and Ketolides ( azithromycin Azithromycin A semi-synthetic macrolide antibiotic structurally related to erythromycin. It has been used in the treatment of Mycobacterium avium intracellulare infections, toxoplasmosis, and cryptosporidiosis. Macrolides and Ketolides), rifampin Rifampin A semisynthetic antibiotic produced from streptomyces mediterranei. It has a broad antibacterial spectrum, including activity against several forms of Mycobacterium. In susceptible organisms it inhibits dna-dependent RNA polymerase activity by forming a stable complex with the enzyme. It thus suppresses the initiation of RNA synthesis. Rifampin is bactericidal, and acts on both intracellular and extracellular organisms. Epiglottitis, and ethambutol.
Last updated: Dec 22, 2022
Antimycobacterial agents represent a diverse group of compounds used against mycobacterial infections Infections Invasion of the host organism by microorganisms or their toxins or by parasites that can cause pathological conditions or diseases. Chronic Granulomatous Disease (e.g., TB TB Tuberculosis (TB) is an infectious disease caused by Mycobacterium tuberculosis complex bacteria. The bacteria usually attack the lungs but can also damage other parts of the body. Approximately 30% of people around the world are infected with this pathogen, with the majority harboring a latent infection. Tuberculosis spreads through the air when a person with active pulmonary infection coughs or sneezes. Tuberculosis, leprosy Leprosy Leprosy, also known as Hansen’s disease, is a chronic bacterial infection caused by Mycobacterium leprae complex bacteria. Symptoms primarily affect the skin and peripheral nerves, resulting in cutaneous manifestations (e.g., hypopigmented macules) and neurologic manifestations (e.g., loss of sensation). Leprosy, Mycobacterium avium Mycobacterium avium A bacterium causing tuberculosis in domestic fowl and other birds. In pigs, it may cause localized and sometimes disseminated disease. The organism occurs occasionally in sheep and cattle. It should be distinguished from the m. avium complex, which infects primarily humans. Mycobacterium complex).
| Bacteria Bacteria Bacteria are prokaryotic single-celled microorganisms that are metabolically active and divide by binary fission. Some of these organisms play a significant role in the pathogenesis of diseases. Bacteriology | Treatment regimen* | Prophylaxis Prophylaxis Cephalosporins |
|---|---|---|
| Mycobacterium tuberculosis Mycobacterium tuberculosis Tuberculosis (TB) is an infectious disease caused by Mycobacterium tuberculosis complex bacteria. The bacteria usually attack the lungs but can also damage other parts of the body. Approximately 30% of people around the world are infected with this pathogen, with the majority harboring a latent infection. Tuberculosis spreads through the air when a person with active pulmonary infection coughs or sneezes. Tuberculosis |
|
Isoniazid |
| M. leprae |
|
None |
M. avium complex (MAC) predominant species within the complex include:
|
|
Azithromycin Azithromycin A semi-synthetic macrolide antibiotic structurally related to erythromycin. It has been used in the treatment of Mycobacterium avium intracellulare infections, toxoplasmosis, and cryptosporidiosis. Macrolides and Ketolides or rifabutin Rifabutin A broad-spectrum antibiotic that is being used as prophylaxis against disseminated Mycobacterium avium complex infection in HIV-positive patients. Diseases of the Uvea |
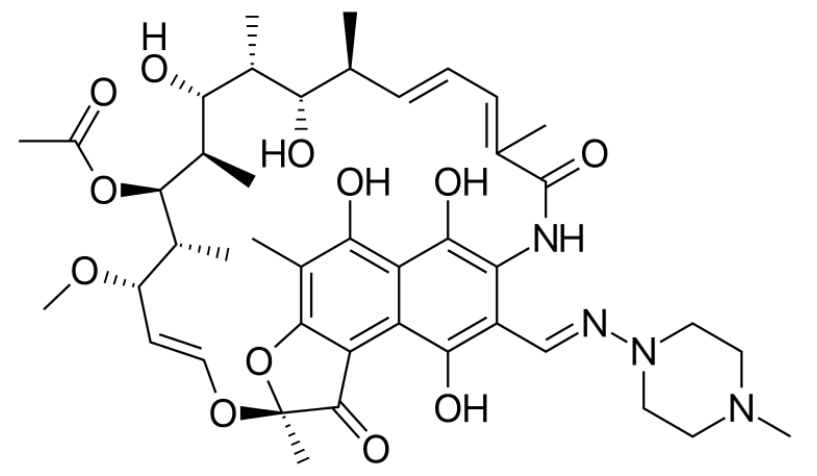
Skeletal formula of rifampin
Image: “Skeletal formula of rifampin” by Vaccinationist. License: Public Domain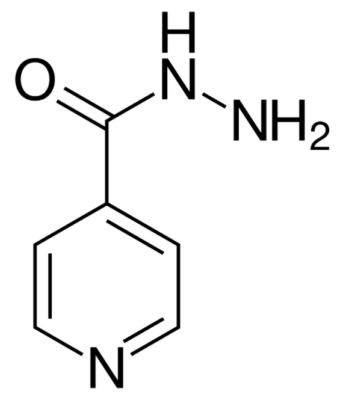
Skeletal formula of isoniazid (isonicotinic acid hydrazine (INH))
Image: “Skeletal formula of isoniazid” by Fvasconcellos. License: Public Domain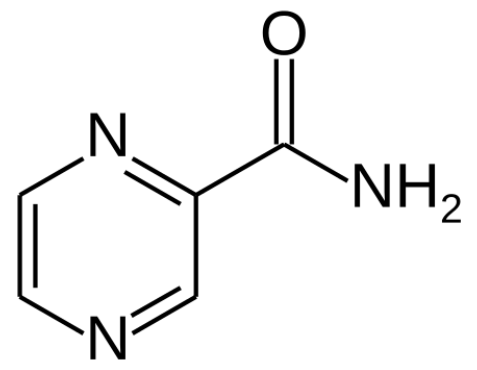
Structure of pyrazinamide
Image: “Structure of pyrazinamide” by Fvasconcellos. License: Public Domain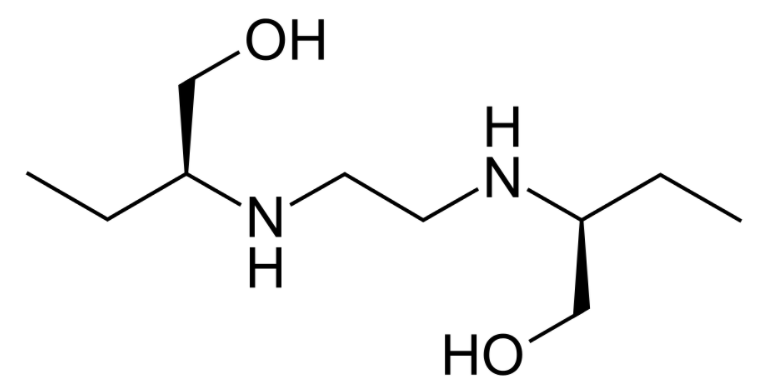
Structure of ethambutol
Image: “Structure of ethambutol” by Fvasconcellos. License: Public DomainAluminum hydroxide ↓ drug absorption Absorption Absorption involves the uptake of nutrient molecules and their transfer from the lumen of the GI tract across the enterocytes and into the interstitial space, where they can be taken up in the venous or lymphatic circulation. Digestion and Absorption
Other agents are used depending on the underlying conditions and presence of multidrug-resistant Mycobacterium Mycobacterium Mycobacterium is a genus of the family Mycobacteriaceae in the phylum Actinobacteria. Mycobacteria comprise more than 150 species of facultative intracellular bacilli that are mostly obligate aerobes. Mycobacteria are responsible for multiple human infections including serious diseases, such as tuberculosis (M. tuberculosis), leprosy (M. leprae), and M. avium complex infections. Mycobacterium.
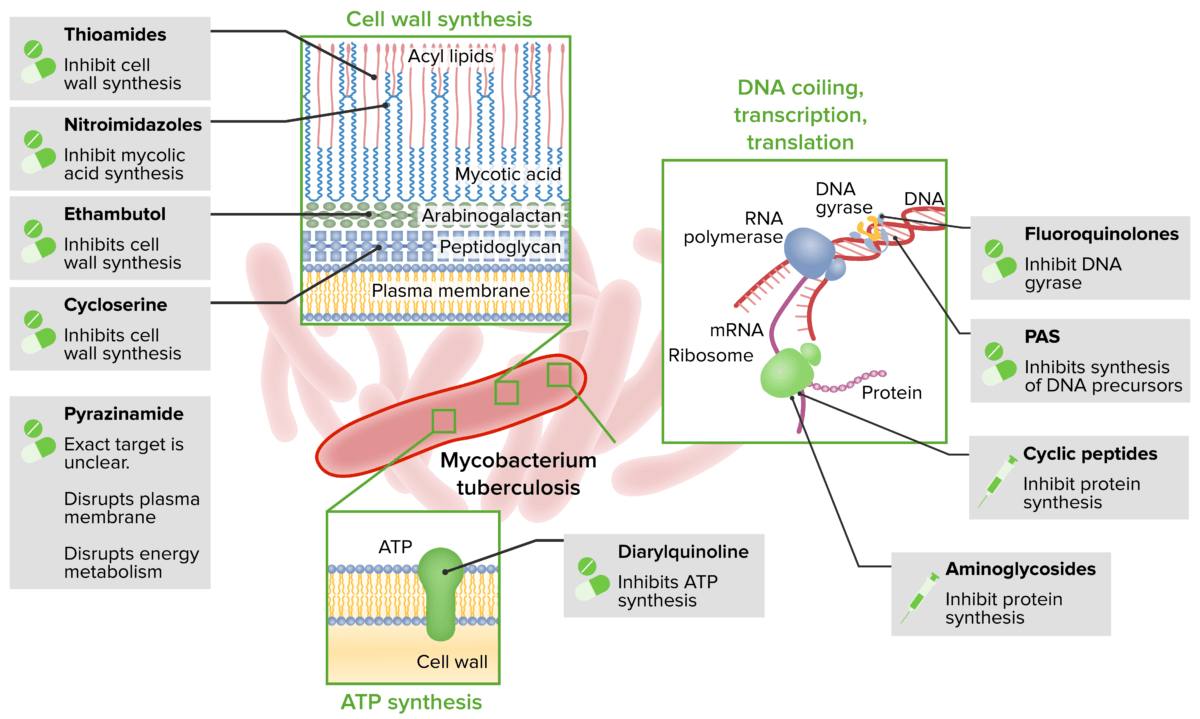
Antituberculosis agents and mechanisms
PAS: Para-aminosalicylic acid