The oxazolidinones (linezolid and tedizolid) are bacterial protein synthesis Synthesis Polymerase Chain Reaction (PCR) inhibitors. The unique binding site on the 23S ribosomal RNA Ribosomal RNA The most abundant form of RNA. Together with proteins, it forms the ribosomes, playing a structural role and also a role in ribosomal binding of mRNA and tRNAs. Individual chains are conventionally designated by their sedimentation coefficients. In eukaryotes, four large chains exist, synthesized in the nucleolus and constituting about 50% of the ribosome. RNA Types and Structure of the 50S ribosome prevents bacteria Bacteria Bacteria are prokaryotic single-celled microorganisms that are metabolically active and divide by binary fission. Some of these organisms play a significant role in the pathogenesis of diseases. Bacteriology from developing cross-resistance when exposed to other antibiotics. These medications are readily absorbed and widely distributed in the body, and they are indicated for gram-positive Gram-Positive Penicillins infections Infections Invasion of the host organism by microorganisms or their toxins or by parasites that can cause pathological conditions or diseases. Chronic Granulomatous Disease, including MRSA MRSA A strain of Staphylococcus aureus that is non-susceptible to the action of methicillin. The mechanism of resistance usually involves modification of normal or the presence of acquired penicillin binding proteins. Staphylococcus and vancomycin-resistant Enterococcus Enterococcus Enterococcus is a genus of oval-shaped gram-positive cocci that are arranged in pairs or short chains. Distinguishing factors include optochin resistance and the presence of pyrrolidonyl arylamidase (PYR) and Lancefield D antigen. Enterococcus is part of the normal flora of the human GI tract. Enterococcus. Adverse effects include GI upset, myelosuppression, peripheral or optic neuropathy Neuropathy Leprosy, and lactic acidosis Acidosis A pathologic condition of acid accumulation or depletion of base in the body. The two main types are respiratory acidosis and metabolic acidosis, due to metabolic acid build up. Respiratory Acidosis. Linezolid is a weak monoamine oxidase Oxidase Neisseria inhibitor, which can increase the risk for serotonin Serotonin A biochemical messenger and regulator, synthesized from the essential amino acid l-tryptophan. In humans it is found primarily in the central nervous system, gastrointestinal tract, and blood platelets. Serotonin mediates several important physiological functions including neurotransmission, gastrointestinal motility, hemostasis, and cardiovascular integrity. Receptors and Neurotransmitters of the CNS syndrome when coadministered with other serotonergic medications.
Last updated: Feb 13, 2025
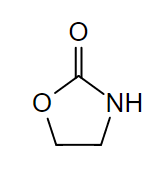
Chemical structure of 2-oxazolidinones:
Linezolid and tedizolid both contain this ring within their chemical structures.
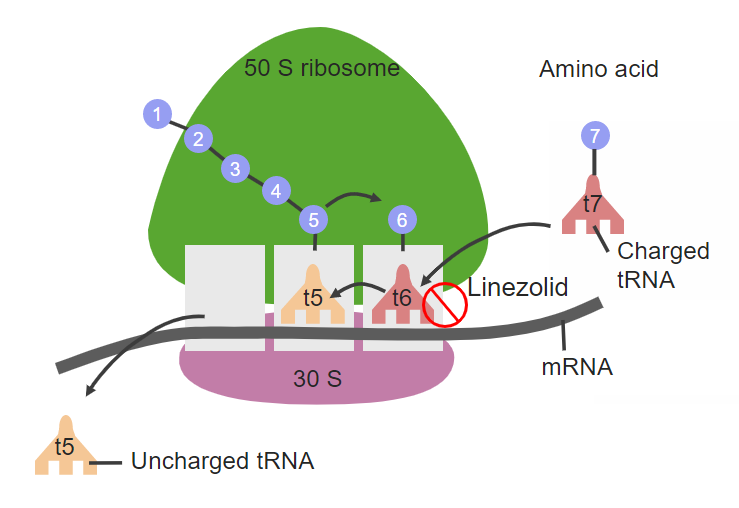
Linezolid site of action on the 50S ribosomal subunit:
This construction inhibits the initiation of protein synthesis and prevents bacterial replication.
These medications are FDA-approved for:
Linezolid may cause a hypertensive crisis in the following conditions:
| Drug class | Mechanism of action | Coverage | Adverse effects |
|---|---|---|---|
| Amphenicols |
|
|
|
| Lincosamides Lincosamides The lincosamides, lincomycin and clindamycin, are inhibitors of bacterial protein synthesis. Drugs in this class share the same binding site as that of macrolides and amphenicols; however, they differ in chemical structure. Lincosamides target the 50S ribosomal subunit and interfere with transpeptidation. Lincosamides |
|
|
|
| Macrolides Macrolides Macrolides and ketolides are antibiotics that inhibit bacterial protein synthesis by binding to the 50S ribosomal subunit and blocking transpeptidation. These antibiotics have a broad spectrum of antimicrobial activity but are best known for their coverage of atypical microorganisms. Macrolides and Ketolides |
|
|
|
| Oxazolidinones |
|
Gram-positive
Gram-Positive
Penicillins
cocci
Cocci
Bacteriology:
|
|
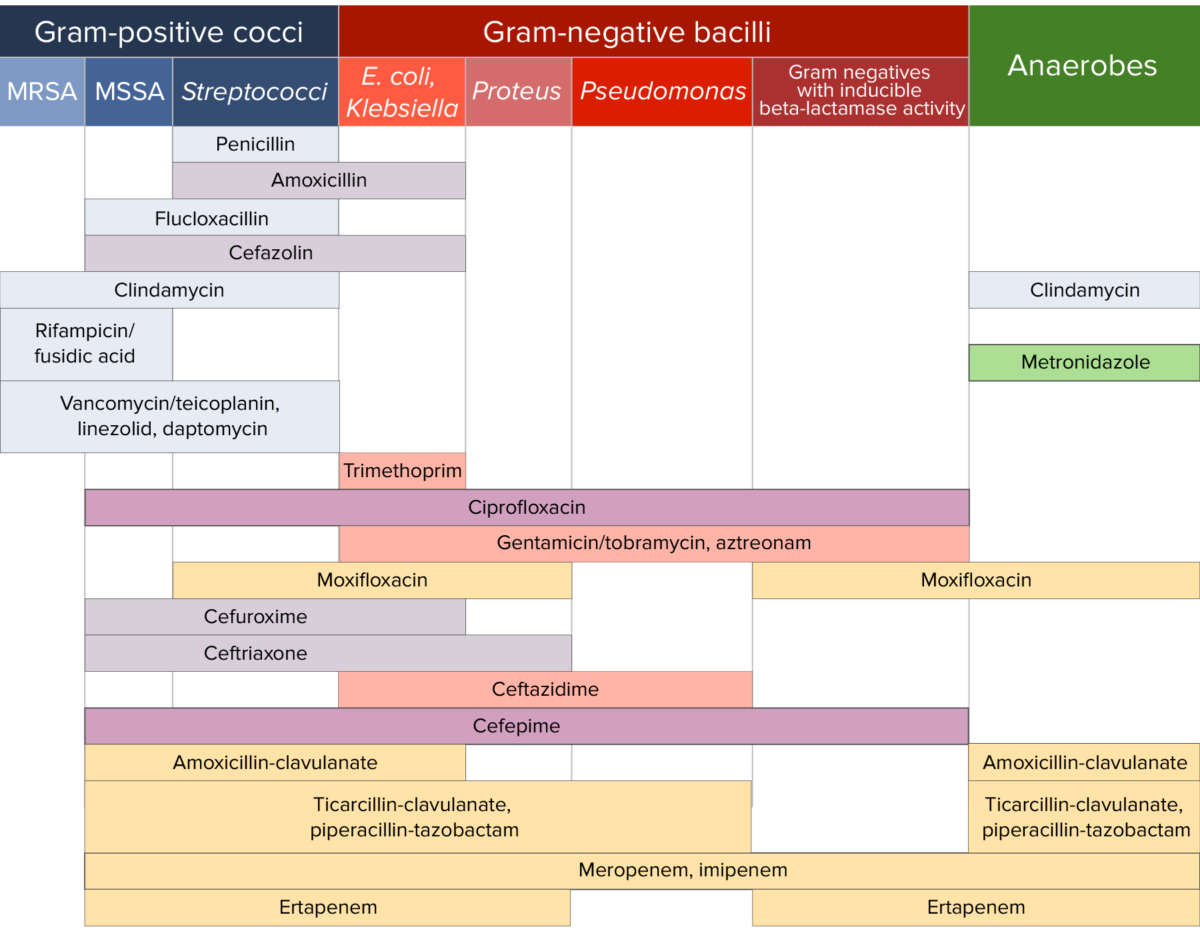
Antibiotic sensitivity:
Chart comparing the microbial coverage of different antibiotics for gram-positive cocci, gram-negative bacilli, and anaerobes.