Myasthenia gravis (MG) is an autoimmune neuromuscular disorder characterized by weakness and fatigability of skeletal muscles Skeletal muscles A subtype of striated muscle, attached by tendons to the skeleton. Skeletal muscles are innervated and their movement can be consciously controlled. They are also called voluntary muscles. Muscle Tissue: Histology caused by dysfunction/destruction of acetylcholine Acetylcholine A neurotransmitter found at neuromuscular junctions, autonomic ganglia, parasympathetic effector junctions, a subset of sympathetic effector junctions, and at many sites in the central nervous system. Receptors and Neurotransmitters of the CNS receptors Receptors Receptors are proteins located either on the surface of or within a cell that can bind to signaling molecules known as ligands (e.g., hormones) and cause some type of response within the cell. Receptors at the neuromuscular junction Neuromuscular junction The synapse between a neuron and a muscle. Skeletal Muscle Contraction. Myasthenia gravis presents with fatigue Fatigue The state of weariness following a period of exertion, mental or physical, characterized by a decreased capacity for work and reduced efficiency to respond to stimuli. Fibromyalgia, ptosis Ptosis Cranial Nerve Palsies, diplopia, dysphagia Dysphagia Dysphagia is the subjective sensation of difficulty swallowing. Symptoms can range from a complete inability to swallow, to the sensation of solids or liquids becoming "stuck." Dysphagia is classified as either oropharyngeal or esophageal, with esophageal dysphagia having 2 sub-types: functional and mechanical. Dysphagia, respiratory difficulties, and progressive weakness in the limbs, leading to difficulty in movement. Diagnosis is established based on clinical presentation, detection of antibodies Antibodies Immunoglobulins (Igs), also known as antibodies, are glycoprotein molecules produced by plasma cells that act in immune responses by recognizing and binding particular antigens. The various Ig classes are IgG (the most abundant), IgM, IgE, IgD, and IgA, which differ in their biologic features, structure, target specificity, and distribution. Immunoglobulins: Types and Functions, and electrophysiologic studies. Management is aimed at increasing the activity of acetylcholine Acetylcholine A neurotransmitter found at neuromuscular junctions, autonomic ganglia, parasympathetic effector junctions, a subset of sympathetic effector junctions, and at many sites in the central nervous system. Receptors and Neurotransmitters of the CNS at the neuromuscular junction Neuromuscular junction The synapse between a neuron and a muscle. Skeletal Muscle Contraction and suppression Suppression Defense Mechanisms of antibodies Antibodies Immunoglobulins (Igs), also known as antibodies, are glycoprotein molecules produced by plasma cells that act in immune responses by recognizing and binding particular antigens. The various Ig classes are IgG (the most abundant), IgM, IgE, IgD, and IgA, which differ in their biologic features, structure, target specificity, and distribution. Immunoglobulins: Types and Functions. This disease can be associated with thymomas and thymic hyperplasia Hyperplasia An increase in the number of cells in a tissue or organ without tumor formation. It differs from hypertrophy, which is an increase in bulk without an increase in the number of cells. Cellular Adaptation, and thymectomy is sometimes indicated. Myasthenia gravis can progress to a life-threatening myasthenic crisis with respiratory failure Respiratory failure Respiratory failure is a syndrome that develops when the respiratory system is unable to maintain oxygenation and/or ventilation. Respiratory failure may be acute or chronic and is classified as hypoxemic, hypercapnic, or a combination of the two. Respiratory Failure, but this is preventable with appropriate management. Prognosis Prognosis A prediction of the probable outcome of a disease based on a individual's condition and the usual course of the disease as seen in similar situations. Non-Hodgkin Lymphomas is generally good with treatment, and some patients Patients Individuals participating in the health care system for the purpose of receiving therapeutic, diagnostic, or preventive procedures. Clinician–Patient Relationship can achieve a long-term remission Remission A spontaneous diminution or abatement of a disease over time, without formal treatment. Cluster Headaches.
Last updated: May 16, 2024
Myasthenia gravis (MG) is a chronic autoimmune disorder Autoimmune Disorder Septic Arthritis in which antibodies Antibodies Immunoglobulins (Igs), also known as antibodies, are glycoprotein molecules produced by plasma cells that act in immune responses by recognizing and binding particular antigens. The various Ig classes are IgG (the most abundant), IgM, IgE, IgD, and IgA, which differ in their biologic features, structure, target specificity, and distribution. Immunoglobulins: Types and Functions attack the acetylcholine Acetylcholine A neurotransmitter found at neuromuscular junctions, autonomic ganglia, parasympathetic effector junctions, a subset of sympathetic effector junctions, and at many sites in the central nervous system. Receptors and Neurotransmitters of the CNS receptor Receptor Receptors are proteins located either on the surface of or within a cell that can bind to signaling molecules known as ligands (e.g., hormones) and cause some type of response within the cell. Receptors (AChR) complex at the neuromuscular junction Neuromuscular junction The synapse between a neuron and a muscle. Skeletal Muscle Contraction.
There are 2 main clinical forms of MG:
In addition, rarer forms of MG exist that primarily affect the pediatric population:
Myasthenia gravis may also be classified on the basis of the serologic profile of antibodies Antibodies Immunoglobulins (Igs), also known as antibodies, are glycoprotein molecules produced by plasma cells that act in immune responses by recognizing and binding particular antigens. The various Ig classes are IgG (the most abundant), IgM, IgE, IgD, and IgA, which differ in their biologic features, structure, target specificity, and distribution. Immunoglobulins: Types and Functions present:
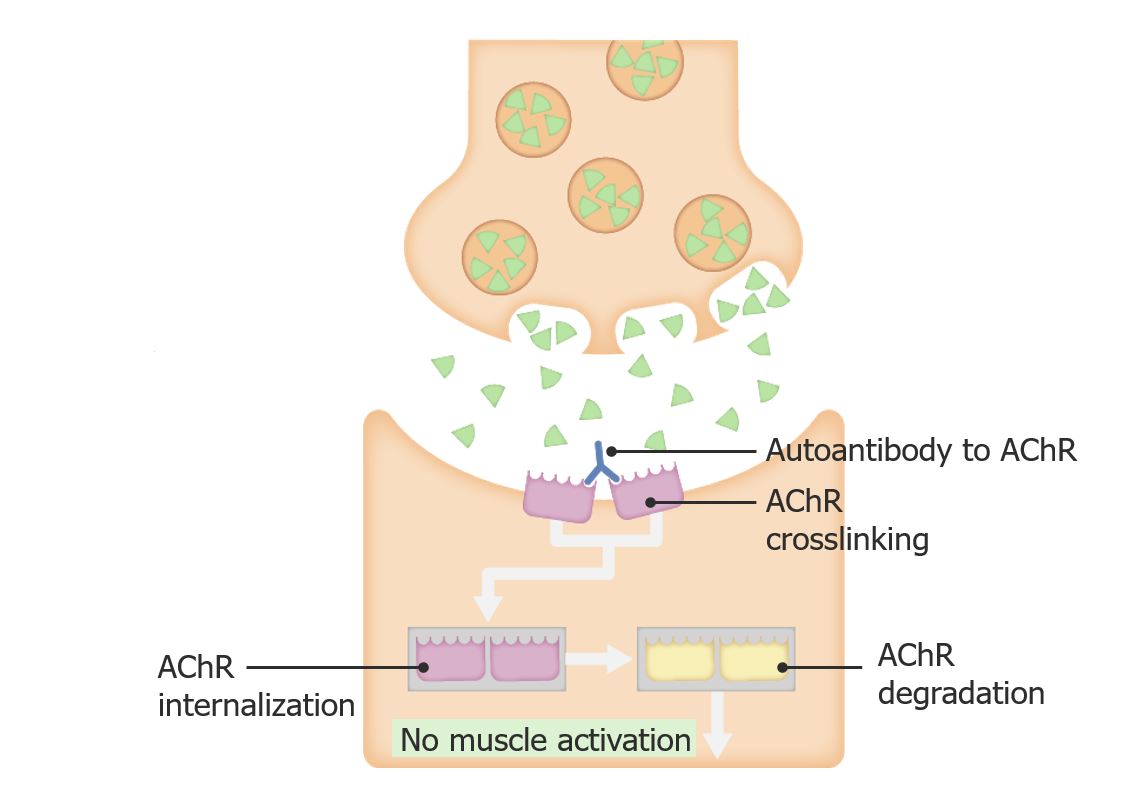
Pathophysiology of myasthenia gravis
Image by Lecturio.
Ptosis in a patient with myasthenia gravis
Image: “Myasthenia Gravis” by Posey & Spiller. License: Public DomainMRI or CT of the chest is done to rule out the possibility of a thymoma Thymoma A neoplasm originating from thymic tissue, usually benign, and frequently encapsulated. Although it is occasionally invasive, metastases are extremely rare. It consists of any type of thymic epithelial cell as well as lymphocytes that are usually abundant. Malignant lymphomas that involve the thymus, e.g., lymphosarcoma, Hodgkin’s disease (previously termed granulomatous thymoma), should not be regarded as thymoma. Primary Lymphatic Organs.
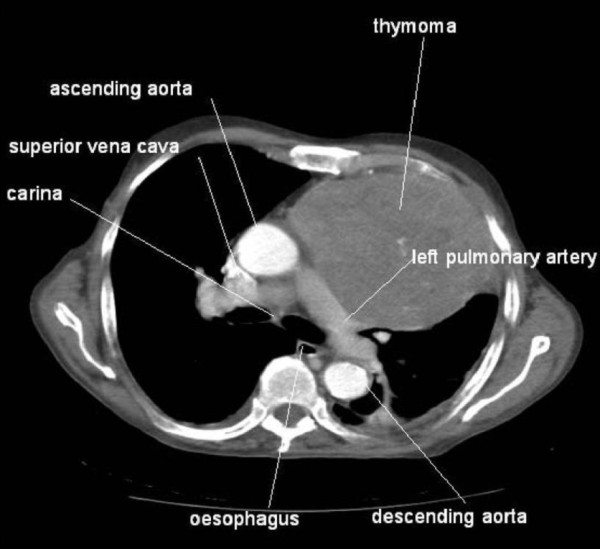
CT scan showing a large thymoma
Image: “Association between thymoma and persistent hypothermia: a case report” by Johns RH, Reinhardt AK. License: CC BY 2.0Acetylcholinesterase inhibitors:
Chronic immunosuppressive drugs Immunosuppressive drugs Agents that suppress immune function by one of several mechanisms of action. Classical cytotoxic immunosuppressants act by inhibiting DNA synthesis. Others may act through activation of T-cells or by inhibiting the activation of helper cells. While immunosuppression has been brought about in the past primarily to prevent rejection of transplanted organs, new applications involving mediation of the effects of interleukins and other cytokines are emerging. Organ Transplantation: