Ebolavirus and Marburgvirus are members of the Filoviridae family. They are single-stranded, negative-sense RNA Negative-sense RNA RNA viruses that have their genetic material encoded in the form of single-stranded, negative-sense RNA. Unlike retroviruses they do not employ DNA intermediates in their life-cycle. Respiratory Syncytial Virus viruses Viruses Minute infectious agents whose genomes are composed of DNA or RNA, but not both. They are characterized by a lack of independent metabolism and the inability to replicate outside living host cells. Virology with a characteristic filamentous, pleomorphic Pleomorphic Bacteroides morphology. Transmission mainly occurs through contact with secretions from an infected individual. These viruses Viruses Minute infectious agents whose genomes are composed of DNA or RNA, but not both. They are characterized by a lack of independent metabolism and the inability to replicate outside living host cells. Virology cause similar diseases, with flu-like symptoms Flu-Like Symptoms Babesia/Babesiosis, diarrhea Diarrhea Diarrhea is defined as ≥ 3 watery or loose stools in a 24-hour period. There are a multitude of etiologies, which can be classified based on the underlying mechanism of disease. The duration of symptoms (acute or chronic) and characteristics of the stools (e.g., watery, bloody, steatorrheic, mucoid) can help guide further diagnostic evaluation. Diarrhea, hemorrhage, multiorgan dysfunction, and shock Shock Shock is a life-threatening condition associated with impaired circulation that results in tissue hypoxia. The different types of shock are based on the underlying cause: distributive (↑ cardiac output (CO), ↓ systemic vascular resistance (SVR)), cardiogenic (↓ CO, ↑ SVR), hypovolemic (↓ CO, ↑ SVR), obstructive (↓ CO), and mixed. Types of Shock. The diagnosis can be made using PCR PCR Polymerase chain reaction (PCR) is a technique that amplifies DNA fragments exponentially for analysis. The process is highly specific, allowing for the targeting of specific genomic sequences, even with minuscule sample amounts. The PCR cycles multiple times through 3 phases: denaturation of the template DNA, annealing of a specific primer to the individual DNA strands, and synthesis/elongation of new DNA molecules. Polymerase Chain Reaction (PCR), antigen detection Antigen detection Respiratory Syncytial Virus, and serology Serology The study of serum, especially of antigen-antibody reactions in vitro. Yellow Fever Virus. Management is mainly supportive, although monoclonal antibody therapy has been promising for treating Ebola virus Virus Viruses are infectious, obligate intracellular parasites composed of a nucleic acid core surrounded by a protein capsid. Viruses can be either naked (non-enveloped) or enveloped. The classification of viruses is complex and based on many factors, including type and structure of the nucleoid and capsid, the presence of an envelope, the replication cycle, and the host range. Virology disease.
Last updated: Oct 31, 2022
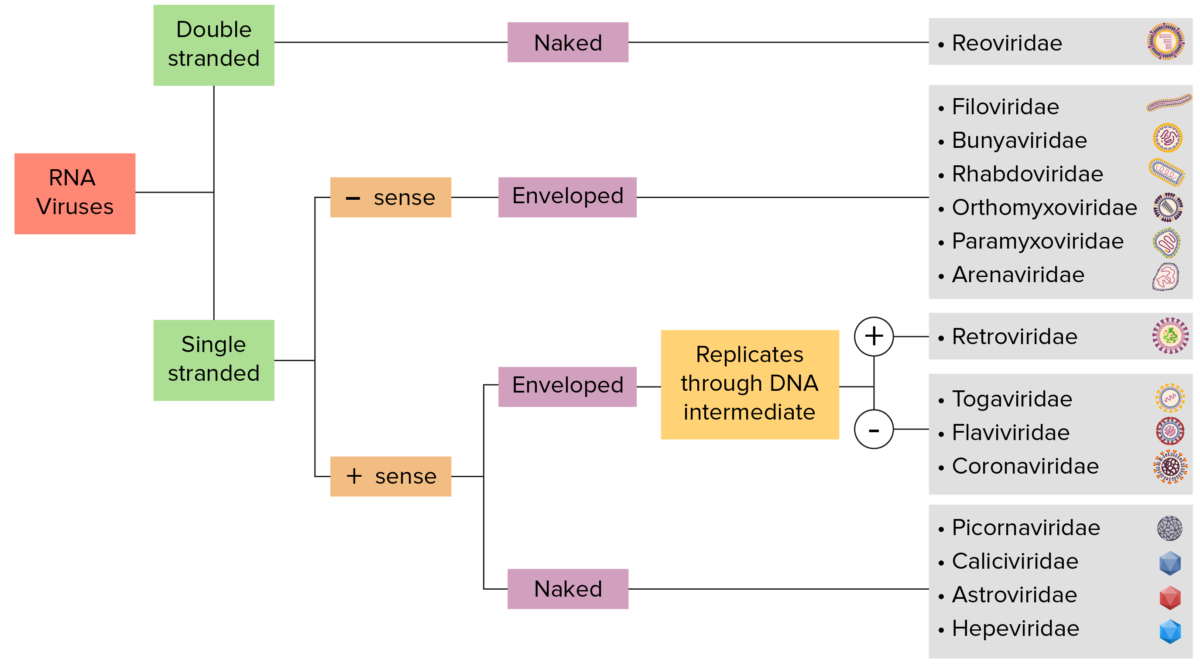
RNA virus identification:
Viruses can be classified in many ways. Most viruses, however, will have a genome formed by either DNA or RNA. RNA genome viruses can be further characterized by either a single- or double-stranded RNA. “Enveloped” viruses are covered by a thin coat of cell membrane (usually taken from the host cell). If the coat is absent, the viruses are called “naked” viruses. Viruses with single-stranded genomes are “positive-sense” viruses if the genome is directly used as messenger RNA (mRNA), which is translated into proteins. “Negative-sense,” single-stranded viruses use RNA-dependent RNA polymerase, a viral enzyme, to transcribe their genome into messenger RNA.
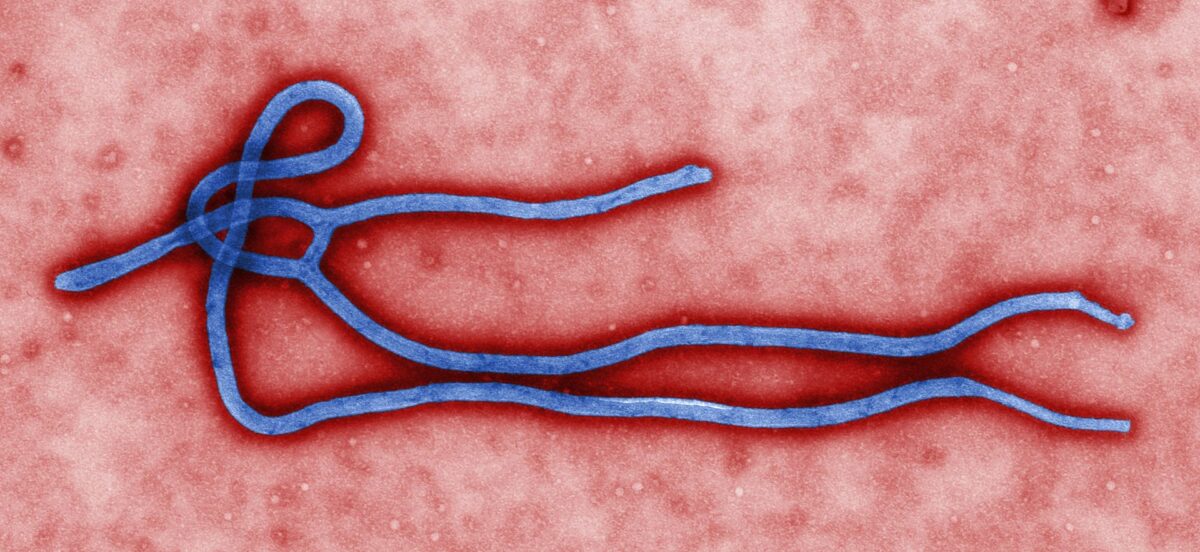
Electron micrograph of an Ebola virus virion:
Note the filamentous structure.
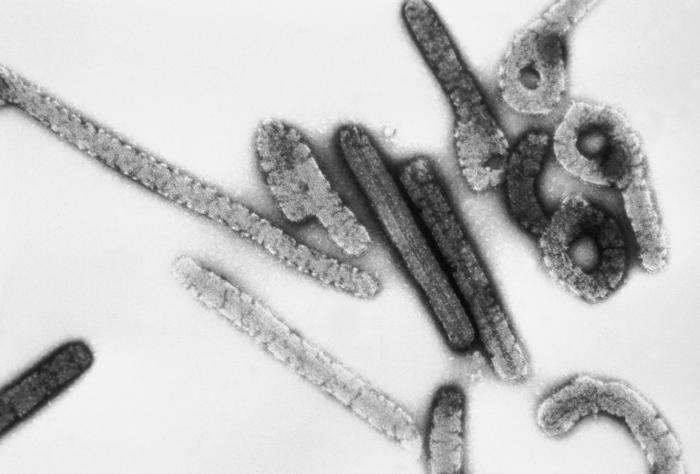
Electron micrograph of Marburg virus virions
Image: “Marburg virus” by Erskine Palmer, Russell Regnery. License: Public Domain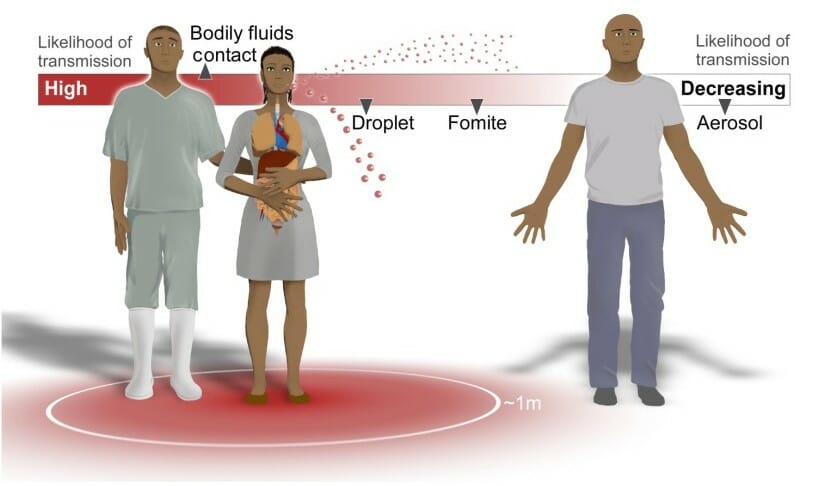
Routes of Ebola virus transmission:
Contact with bodily fluids such as blood, stool, semen, breast milk, and saliva carry the highest risk of transmission. The infectious fluids can also be formed in aerosol droplets, which carry a lower risk of transmission.
Ebola virus Virus Viruses are infectious, obligate intracellular parasites composed of a nucleic acid core surrounded by a protein capsid. Viruses can be either naked (non-enveloped) or enveloped. The classification of viruses is complex and based on many factors, including type and structure of the nucleoid and capsid, the presence of an envelope, the replication cycle, and the host range. Virology disease and MVD have very similar presentations and can be difficult to distinguish.
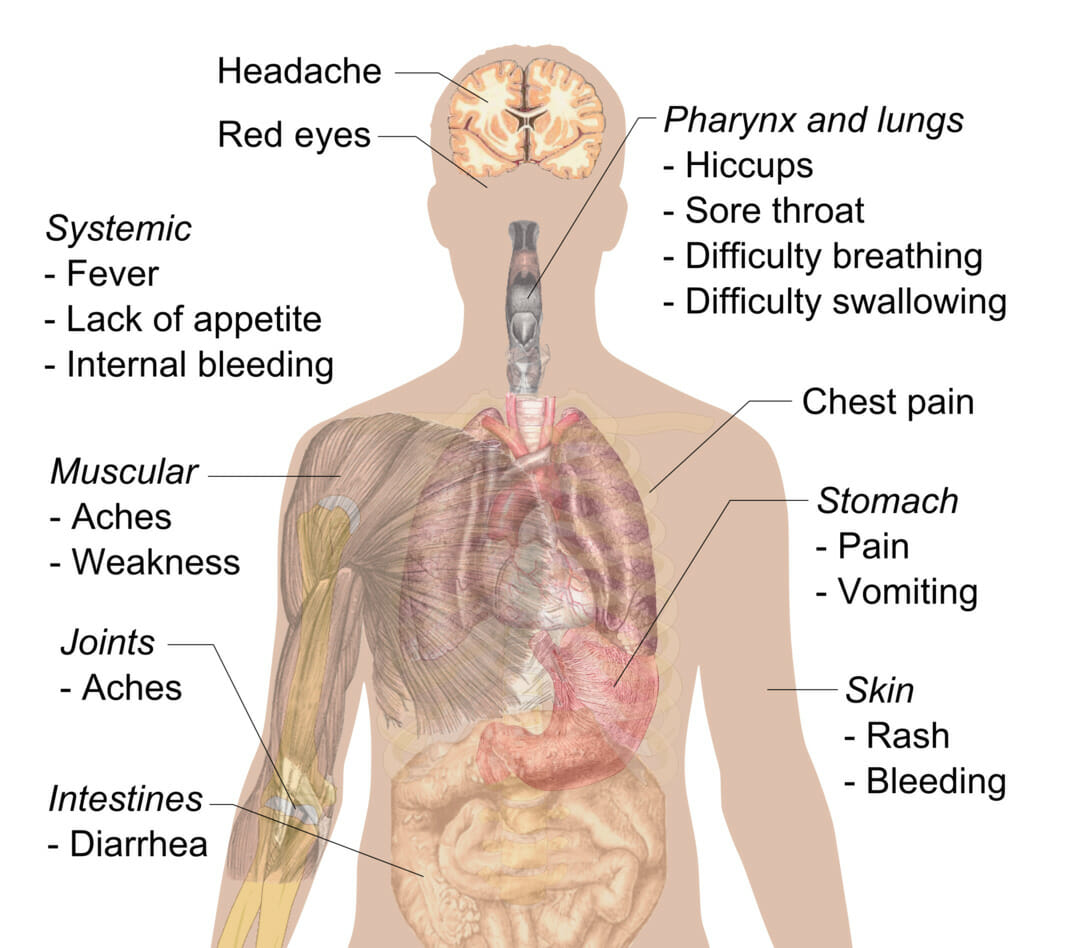
Signs and symptoms of Ebola
Image: “Symptoms of ebola” by Mikael Häggström. License: CC0 1.0, cropped by Lecturio.Diagnosing EVD and MVD early in the course can be difficult (early symptoms mimic other more common diseases). A high degree of suspicion is required, as early isolation of patients Patients Individuals participating in the health care system for the purpose of receiving therapeutic, diagnostic, or preventive procedures. Clinician–Patient Relationship in whom EVD or MVD is suspected is essential to control an outbreak.
The following table compares and contrasts several viral causes of hemorrhagic fever Fever Fever is defined as a measured body temperature of at least 38°C (100.4°F). Fever is caused by circulating endogenous and/or exogenous pyrogens that increase levels of prostaglandin E2 in the hypothalamus. Fever is commonly associated with chills, rigors, sweating, and flushing of the skin. Fever:
| Organism | Ebola virus Virus Viruses are infectious, obligate intracellular parasites composed of a nucleic acid core surrounded by a protein capsid. Viruses can be either naked (non-enveloped) or enveloped. The classification of viruses is complex and based on many factors, including type and structure of the nucleoid and capsid, the presence of an envelope, the replication cycle, and the host range. Virology | Yellow fever Fever Fever is defined as a measured body temperature of at least 38°C (100.4°F). Fever is caused by circulating endogenous and/or exogenous pyrogens that increase levels of prostaglandin E2 in the hypothalamus. Fever is commonly associated with chills, rigors, sweating, and flushing of the skin. Fever virus Virus Viruses are infectious, obligate intracellular parasites composed of a nucleic acid core surrounded by a protein capsid. Viruses can be either naked (non-enveloped) or enveloped. The classification of viruses is complex and based on many factors, including type and structure of the nucleoid and capsid, the presence of an envelope, the replication cycle, and the host range. Virology | Hantavirus Hantavirus A genus of the family bunyaviridae causing hantavirus infections, first identified during the korean war. Infection is found primarily in rodents and humans. Transmission does not appear to involve arthropods. Hantaan virus is the type species. Bunyavirales | Lassa virus Lassa Virus Lassa virus, part of the Arenaviridae family, is an ssRNA virus that causes Lassa fever, a type of viral hemorrhagic illness. The virus is endemic in parts of West Africa (Sierra Leone, Liberia, Guinea, and Nigeria) and neighboring countries. The reservoir is the multimammate rat (Mastomys natalensis), and transmission is via inhalation or contact with rodent excretions or consumption of rodents. Lassa Virus |
|---|---|---|---|---|
| Family | Filoviridae | Flaviviridae Flaviviridae A family of RNA viruses, many of which cause disease in humans and domestic animals. There are three genera flavivirus; pestivirus; and hepacivirus, as well as several unassigned species. Hepatitis C Virus | Bunyaviridae Bunyaviridae A family of viruses, mainly arboviruses, consisting of a single strand of RNA. Virions are enveloped particles 90-120 nm diameter. The complete family contains over 300 members arranged in five genera: orthobunyavirus; hantavirus; nairovirus; phlebovirus; and tospovirus. Bunyavirales | Arenaviridae Arenaviridae A family of RNA viruses naturally infecting rodents and consisting of one genus (arenavirus) with two groups: old world arenaviruses and new world arenaviruses. Infection in rodents is persistent and silent. Vertical transmission is through milk-, saliva-, or urine-borne routes. Horizontal transmission to humans, monkeys, and other animals is important. Lassa Virus |
| Characteristics |
|
|
|
|
| Transmission |
|
Vector: mosquito |
|
|
| Clinical presentation |
|
|
|
|
| Diagnosis |
|
|
|
|
| Management |
|
Supportive | Supportive |
|