Lassa virus Virus Viruses are infectious, obligate intracellular parasites composed of a nucleic acid core surrounded by a protein capsid. Viruses can be either naked (non-enveloped) or enveloped. The classification of viruses is complex and based on many factors, including type and structure of the nucleoid and capsid, the presence of an envelope, the replication cycle, and the host range. Virology, part of the Arenaviridae family, is an ssRNA virus Virus Viruses are infectious, obligate intracellular parasites composed of a nucleic acid core surrounded by a protein capsid. Viruses can be either naked (non-enveloped) or enveloped. The classification of viruses is complex and based on many factors, including type and structure of the nucleoid and capsid, the presence of an envelope, the replication cycle, and the host range. Virology that causes Lassa fever Fever Fever is defined as a measured body temperature of at least 38°C (100.4°F). Fever is caused by circulating endogenous and/or exogenous pyrogens that increase levels of prostaglandin E2 in the hypothalamus. Fever is commonly associated with chills, rigors, sweating, and flushing of the skin. Fever, a type of viral hemorrhagic illness. The virus Virus Viruses are infectious, obligate intracellular parasites composed of a nucleic acid core surrounded by a protein capsid. Viruses can be either naked (non-enveloped) or enveloped. The classification of viruses is complex and based on many factors, including type and structure of the nucleoid and capsid, the presence of an envelope, the replication cycle, and the host range. Virology is endemic in parts of West Africa (Sierra Leone, Liberia, Guinea, and Nigeria) and neighboring countries. The reservoir Reservoir Animate or inanimate sources which normally harbor disease-causing organisms and thus serve as potential sources of disease outbreaks. Reservoirs are distinguished from vectors (disease vectors) and carriers, which are agents of disease transmission rather than continuing sources of potential disease outbreaks. Humans may serve both as disease reservoirs and carriers. Escherichia coli is the multimammate rat (Mastomys natalensis), and transmission is via inhalation or contact with rodent excretions or consumption of rodents. Human-to-human transmission also occurs. The majority of infected patients Patients Individuals participating in the health care system for the purpose of receiving therapeutic, diagnostic, or preventive procedures. Clinician–Patient Relationship are asymptomatic. However, symptomatic patients Patients Individuals participating in the health care system for the purpose of receiving therapeutic, diagnostic, or preventive procedures. Clinician–Patient Relationship present with fever Fever Fever is defined as a measured body temperature of at least 38°C (100.4°F). Fever is caused by circulating endogenous and/or exogenous pyrogens that increase levels of prostaglandin E2 in the hypothalamus. Fever is commonly associated with chills, rigors, sweating, and flushing of the skin. Fever, malaise Malaise Tick-borne Encephalitis Virus, headaches, vomiting Vomiting The forcible expulsion of the contents of the stomach through the mouth. Hypokalemia, and chest pains, which can progress to hemorrhage, hypovolemia Hypovolemia Sepsis in Children, and shock Shock Shock is a life-threatening condition associated with impaired circulation that results in tissue hypoxia. The different types of shock are based on the underlying cause: distributive (↑ cardiac output (CO), ↓ systemic vascular resistance (SVR)), cardiogenic (↓ CO, ↑ SVR), hypovolemic (↓ CO, ↑ SVR), obstructive (↓ CO), and mixed. Types of Shock. Treatment is mainly with ribavirin Ribavirin A nucleoside antimetabolite antiviral agent that blocks nucleic acid synthesis and is used against both RNA and DNA viruses. Antivirals for Hepatitis C and supportive care. There is no effective vaccine Vaccine Suspensions of killed or attenuated microorganisms (bacteria, viruses, fungi, protozoa), antigenic proteins, synthetic constructs, or other bio-molecular derivatives, administered for the prevention, amelioration, or treatment of infectious and other diseases. Vaccination, but preventive measures such as avoiding rodent exposures and use of PPE can reduce transmission. Ribavirin Ribavirin A nucleoside antimetabolite antiviral agent that blocks nucleic acid synthesis and is used against both RNA and DNA viruses. Antivirals for Hepatitis C is also given as postexposure prophylaxis Prophylaxis Cephalosporins.
Last updated: Mar 13, 2025
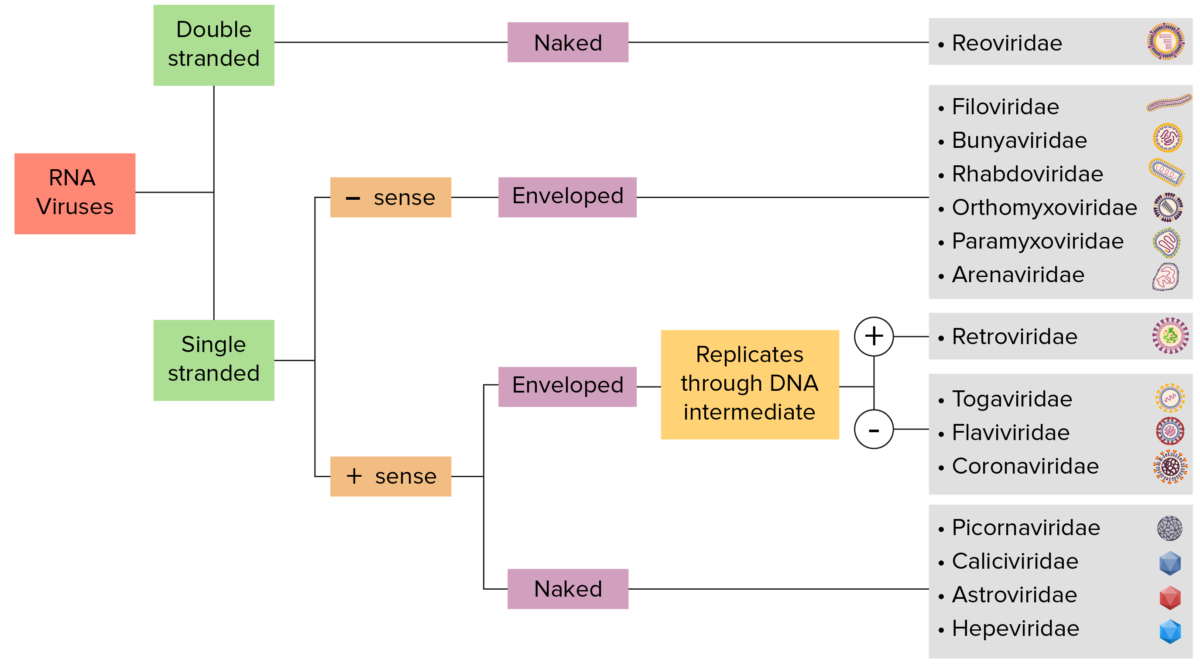
RNA virus identification:
Viruses can be classified in many ways. Most viruses, however, will have a genome formed by either DNA or RNA. RNA genome viruses can be further characterized by either a single- or double-stranded RNA. “Enveloped” viruses are covered by a thin coat of cell membrane (usually taken from the host cell). If the coat is absent, the viruses are called “naked” viruses. Viruses with single-stranded genomes are “positive-sense” viruses if the genome is directly employed as messenger RNA (mRNA), which is translated into proteins. “Negative-sense,” single-stranded viruses employ RNA dependent RNA polymerase, a viral enzyme, to transcribe their genome into messenger RNA.
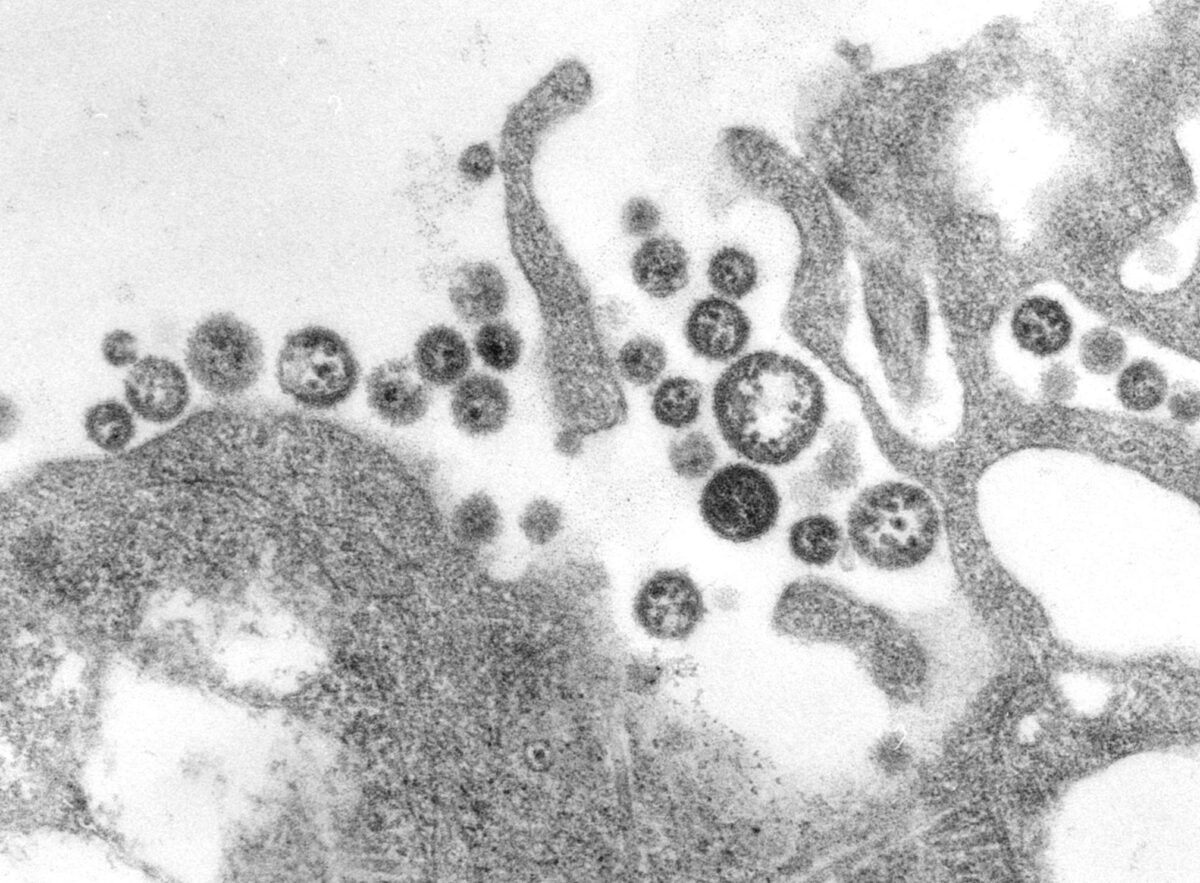
An electron micrograph of Lassa virus virions
Image: “A transmission electron micrograph (TEM) of a number of Lassa virus virions adjacent to some cell debris” by CDC/C. S. Goldsmith. License: Public Domain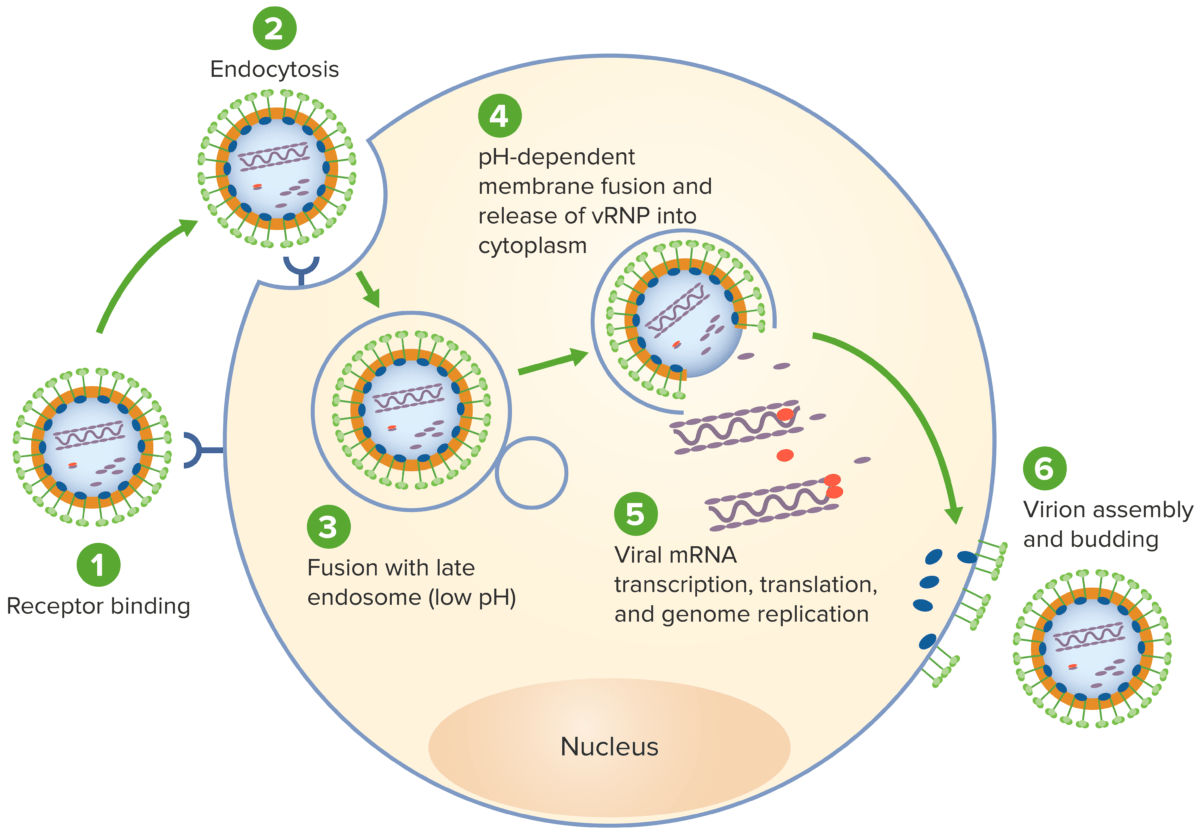
The life cycle of lassa virus:
1. Virus attaches to host receptors through envelope glycoproteins.
2. Virus is subsequently endocytosed, thus gaining cell entry.
3. Viral fusion with an endosome follows.
4. The virus fuses with the endosome membrane, allowing release of the viral ribonucleocapsid into the cytoplasm.
5. The RNA-dependent RNA polymerase (RdRP) mediates the viral gene replication and transcription in the host cytoplasm.
6. New virions are assembled and budding occurs, releasing the virion to infect other cells.
vRNP: viral ribonucleoprotein
Arenaviruses can cause hemorrhagic fevers (Lassa virus Virus Viruses are infectious, obligate intracellular parasites composed of a nucleic acid core surrounded by a protein capsid. Viruses can be either naked (non-enveloped) or enveloped. The classification of viruses is complex and based on many factors, including type and structure of the nucleoid and capsid, the presence of an envelope, the replication cycle, and the host range. Virology) and/or neurologic disease ( lymphocytic choriomeningitis virus Lymphocytic Choriomeningitis Virus Lymphocytic choriomeningitis virus (LCMV) is a single-stranded RNA virus transmitted to humans via rodents, the primary reservoir. Viral infections can occur through direct contact (such as through a break in the skin) with rodent urine, saliva, or droppings or via inhalation of aerosolized virus. The disease typically results in a self-limited, febrile, biphasic disease. Lymphocytic Choriomeningitis Virus ( LCMV LCMV Lymphocytic choriomeningitis virus (LCMV) is a single-stranded RNA virus transmitted to humans via rodents, the primary reservoir. Viral infections can occur through direct contact (such as through a break in the skin) with rodent urine, saliva, or droppings or via inhalation of aerosolized virus. The disease typically results in a self-limited, febrile, biphasic disease. Lymphocytic Choriomeningitis Virus)).
| Organism | LCMV LCMV Lymphocytic choriomeningitis virus (LCMV) is a single-stranded RNA virus transmitted to humans via rodents, the primary reservoir. Viral infections can occur through direct contact (such as through a break in the skin) with rodent urine, saliva, or droppings or via inhalation of aerosolized virus. The disease typically results in a self-limited, febrile, biphasic disease. Lymphocytic Choriomeningitis Virus | Lassa virus Virus Viruses are infectious, obligate intracellular parasites composed of a nucleic acid core surrounded by a protein capsid. Viruses can be either naked (non-enveloped) or enveloped. The classification of viruses is complex and based on many factors, including type and structure of the nucleoid and capsid, the presence of an envelope, the replication cycle, and the host range. Virology |
|---|---|---|
| Family | Arenaviridae | Arenaviridae |
| Genus | Mammarenavirus | Mammarenavirus |
| Characteristics |
|
|
| Reservoir Reservoir Animate or inanimate sources which normally harbor disease-causing organisms and thus serve as potential sources of disease outbreaks. Reservoirs are distinguished from vectors (disease vectors) and carriers, which are agents of disease transmission rather than continuing sources of potential disease outbreaks. Humans may serve both as disease reservoirs and carriers. Escherichia coli | Rodents | Rodents |
| Transmission |
|
|
| Clinical course | Biphasic:
|
|
| Diagnosis |
|
|
| Management |
|
|
| Prevention |
|
|
Other viral hemorrhagic fevers:
Conditions caused by other nonviral pathogens: