Intestinal hookworm infections Infections Invasion of the host organism by microorganisms or their toxins or by parasites that can cause pathological conditions or diseases. Chronic Granulomatous Disease that affect humans are caused mainly by Necator americanus and Ancylostoma duodenale. Millions of people are infected around the world, mainly in tropical regions, where warm and moist environments facilitate larva survival in the soil. Transmission is via dermal penetration Penetration X-rays by the larvae Larvae Wormlike or grublike stage, following the egg in the life cycle of insects, worms, and other metamorphosing animals. Ascaris/Ascariasis. From entry, the parasite undergoes a transpulmonary passage, reaching the trachea Trachea The trachea is a tubular structure that forms part of the lower respiratory tract. The trachea is continuous superiorly with the larynx and inferiorly becomes the bronchial tree within the lungs. The trachea consists of a support frame of semicircular, or C-shaped, rings made out of hyaline cartilage and reinforced by collagenous connective tissue. Trachea: Anatomy and pharynx Pharynx The pharynx is a component of the digestive system that lies posterior to the nasal cavity, oral cavity, and larynx. The pharynx can be divided into the oropharynx, nasopharynx, and laryngopharynx. Pharyngeal muscles play an integral role in vital processes such as breathing, swallowing, and speaking. Pharynx: Anatomy, where it is swallowed. In the small intestine Small intestine The small intestine is the longest part of the GI tract, extending from the pyloric orifice of the stomach to the ileocecal junction. The small intestine is the major organ responsible for chemical digestion and absorption of nutrients. It is divided into 3 segments: the duodenum, the jejunum, and the ileum. Small Intestine: Anatomy, worms mature and attach to the duodenum Duodenum The shortest and widest portion of the small intestine adjacent to the pylorus of the stomach. It is named for having the length equal to about the width of 12 fingers. Small Intestine: Anatomy. Diarrhea Diarrhea Diarrhea is defined as ≥ 3 watery or loose stools in a 24-hour period. There are a multitude of etiologies, which can be classified based on the underlying mechanism of disease. The duration of symptoms (acute or chronic) and characteristics of the stools (e.g., watery, bloody, steatorrheic, mucoid) can help guide further diagnostic evaluation. Diarrhea, nausea Nausea An unpleasant sensation in the stomach usually accompanied by the urge to vomit. Common causes are early pregnancy, sea and motion sickness, emotional stress, intense pain, food poisoning, and various enteroviruses. Antiemetics, and vomiting Vomiting The forcible expulsion of the contents of the stomach through the mouth. Hypokalemia are GI symptoms. Blood loss (leading to anemia Anemia Anemia is a condition in which individuals have low Hb levels, which can arise from various causes. Anemia is accompanied by a reduced number of RBCs and may manifest with fatigue, shortness of breath, pallor, and weakness. Subtypes are classified by the size of RBCs, chronicity, and etiology. Anemia: Overview and Types) and subsequent malnutrition Malnutrition Malnutrition is a clinical state caused by an imbalance or deficiency of calories and/or micronutrients and macronutrients. The 2 main manifestations of acute severe malnutrition are marasmus (total caloric insufficiency) and kwashiorkor (protein malnutrition with characteristic edema). Malnutrition in children in resource-limited countries are complications. Diagnosis is by stool microscopy Stool Microscopy Giardia/Giardiasis showing the hookworm eggs and by PCR PCR Polymerase chain reaction (PCR) is a technique that amplifies DNA fragments exponentially for analysis. The process is highly specific, allowing for the targeting of specific genomic sequences, even with minuscule sample amounts. The PCR cycles multiple times through 3 phases: denaturation of the template DNA, annealing of a specific primer to the individual DNA strands, and synthesis/elongation of new DNA molecules. Polymerase Chain Reaction (PCR). Management targets prevention through proper sanitation Sanitation The development and establishment of environmental conditions favorable to the health of the public. Hepatitis E Virus and regular Regular Insulin deworming of high-risk groups. Treatment involves the use of anti-parasitic medications, with iron Iron A metallic element with atomic symbol fe, atomic number 26, and atomic weight 55. 85. It is an essential constituent of hemoglobins; cytochromes; and iron-binding proteins. It plays a role in cellular redox reactions and in the transport of oxygen. Trace Elements supplements for anemia Anemia Anemia is a condition in which individuals have low Hb levels, which can arise from various causes. Anemia is accompanied by a reduced number of RBCs and may manifest with fatigue, shortness of breath, pallor, and weakness. Subtypes are classified by the size of RBCs, chronicity, and etiology. Anemia: Overview and Types.
Last updated: Feb 3, 2025
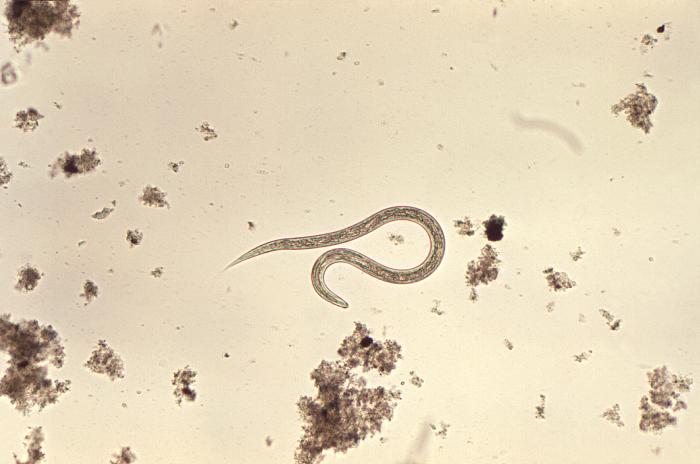
Photomicrograph showing the ultrastructural morphology exhibited by a hookworm filariform larva
Image: “1431” by Dr. Mae Melvin. License: Public Domain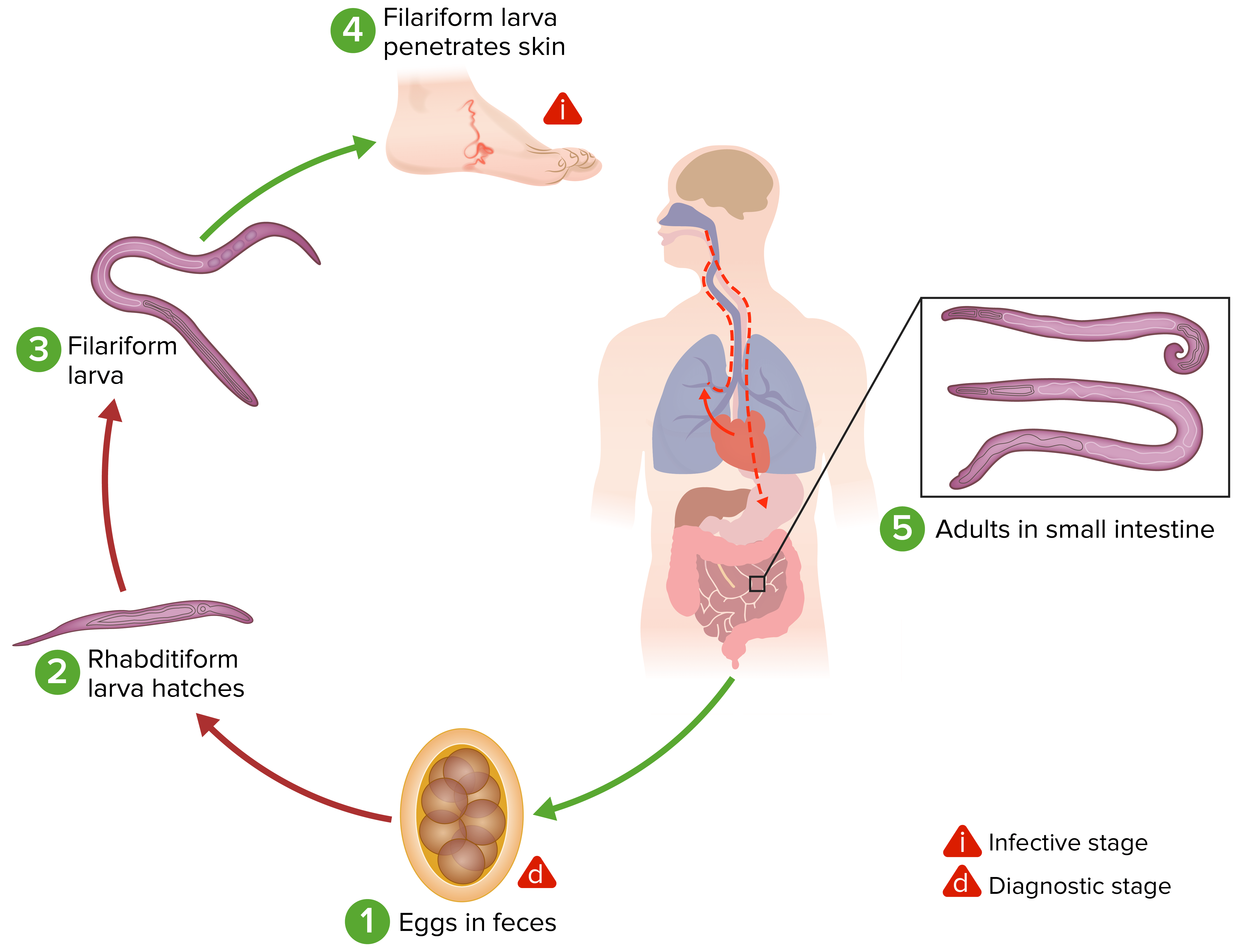
Life cycle of hookworms:
(1) Eggs from stool contaminate the soil. Under favorable conditions (moisture, warmth, shade), larvae hatch in 1–2 days.
(2) The rhabditiform larvae grow in the feces and/or the soil, and (3) subsequently become the infective filariform larvae.
(4) A human host comes in contact with the contaminated soil, the larvae penetrate the skin, and, with circulation, pass through the heart and then to the lungs (pulmonary alveoli). Larvae move up the bronchial tree to the pharynx and are swallowed. The larvae then reach the small bowel, where they will mature into adults.
(5) Adult worms attach to the intestinal wall with resultant blood loss by the host. Most adult worms live up to 1–2 years, even longer in some hosts.
Some A. duodenale larvae can become dormant (in the intestine or muscle) after skin penetration. In addition, infection by A. duodenale may be transmitted by the oral and transmammary route.
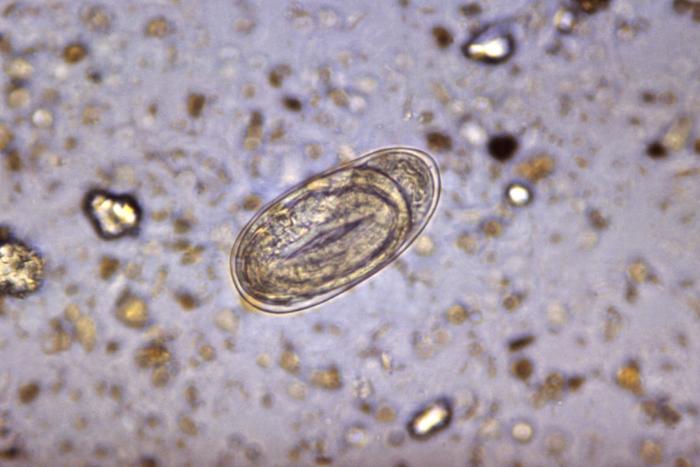
Diagnostic characteristics of hookworm eggs include a thin shell, which is oval or ellipsoidal in shape.
Image: “4825” by CDC. License: Public DomainMedical management:
Prevention: