Anemia is a condition in which individuals have low Hb Hb The oxygen-carrying proteins of erythrocytes. They are found in all vertebrates and some invertebrates. The number of globin subunits in the hemoglobin quaternary structure differs between species. Structures range from monomeric to a variety of multimeric arrangements. Gas Exchange levels, which can arise from various causes. Anemia is accompanied by a reduced number of RBCs RBCs Erythrocytes, or red blood cells (RBCs), are the most abundant cells in the blood. While erythrocytes in the fetus are initially produced in the yolk sac then the liver, the bone marrow eventually becomes the main site of production. Erythrocytes: Histology and may manifest with fatigue Fatigue The state of weariness following a period of exertion, mental or physical, characterized by a decreased capacity for work and reduced efficiency to respond to stimuli. Fibromyalgia, shortness of breath Shortness of breath Dyspnea is the subjective sensation of breathing discomfort. Dyspnea is a normal manifestation of heavy physical or psychological exertion, but also may be caused by underlying conditions (both pulmonary and extrapulmonary). Dyspnea, pallor, and weakness. Subtypes are classified by the size of RBCs RBCs Erythrocytes, or red blood cells (RBCs), are the most abundant cells in the blood. While erythrocytes in the fetus are initially produced in the yolk sac then the liver, the bone marrow eventually becomes the main site of production. Erythrocytes: Histology, chronicity, and etiology. Anemia may occur from blood loss, decreased RBC production such as in iron Iron A metallic element with atomic symbol fe, atomic number 26, and atomic weight 55. 85. It is an essential constituent of hemoglobins; cytochromes; and iron-binding proteins. It plays a role in cellular redox reactions and in the transport of oxygen. Trace Elements deficiency, or increased RBC destruction such as in hemolysis. Management is aimed at improving Hb Hb The oxygen-carrying proteins of erythrocytes. They are found in all vertebrates and some invertebrates. The number of globin subunits in the hemoglobin quaternary structure differs between species. Structures range from monomeric to a variety of multimeric arrangements. Gas Exchange levels and treating the underlying conditions.
Last updated: Jun 30, 2025
Anemia is a quantitative deficiency of Hb Hb The oxygen-carrying proteins of erythrocytes. They are found in all vertebrates and some invertebrates. The number of globin subunits in the hemoglobin quaternary structure differs between species. Structures range from monomeric to a variety of multimeric arrangements. Gas Exchange, the oxygen-carrying component of RBCs RBCs Erythrocytes, or red blood cells (RBCs), are the most abundant cells in the blood. While erythrocytes in the fetus are initially produced in the yolk sac then the liver, the bone marrow eventually becomes the main site of production. Erythrocytes: Histology. Anemia is noted when Hb Hb The oxygen-carrying proteins of erythrocytes. They are found in all vertebrates and some invertebrates. The number of globin subunits in the hemoglobin quaternary structure differs between species. Structures range from monomeric to a variety of multimeric arrangements. Gas Exchange levels are approximately:
Normal levels can vary depending on the laboratory reference range.
Anemia can be caused by:
| Etiology | Categories | Types of anemia |
|---|---|---|
| Blood loss | Acute |
|
| Chronic |
|
|
| Decreased RBC production | Abnormal proliferation or differentiation of stem cells |
|
| Defective DNA DNA A deoxyribonucleotide polymer that is the primary genetic material of all cells. Eukaryotic and prokaryotic organisms normally contain DNA in a double-stranded state, yet several important biological processes transiently involve single-stranded regions. DNA, which consists of a polysugar-phosphate backbone possessing projections of purines (adenine and guanine) and pyrimidines (thymine and cytosine), forms a double helix that is held together by hydrogen bonds between these purines and pyrimidines (adenine to thymine and guanine to cytosine). DNA Types and Structure synthesis Synthesis Polymerase Chain Reaction (PCR) in erythroblasts |
|
|
| Defective Hb Hb The oxygen-carrying proteins of erythrocytes. They are found in all vertebrates and some invertebrates. The number of globin subunits in the hemoglobin quaternary structure differs between species. Structures range from monomeric to a variety of multimeric arrangements. Gas Exchange synthesis Synthesis Polymerase Chain Reaction (PCR) |
|
|
| Unknown or multiple mechanisms |
|
|
| Increased RBC destruction | Abnormal Hb Hb The oxygen-carrying proteins of erythrocytes. They are found in all vertebrates and some invertebrates. The number of globin subunits in the hemoglobin quaternary structure differs between species. Structures range from monomeric to a variety of multimeric arrangements. Gas Exchange |
|
| Enzyme deficiencies |
|
|
| Membrane disorders |
|
|
| Immune-mediated |
|
|
| Mechanical trauma to RBCs RBCs Erythrocytes, or red blood cells (RBCs), are the most abundant cells in the blood. While erythrocytes in the fetus are initially produced in the yolk sac then the liver, the bone marrow eventually becomes the main site of production. Erythrocytes: Histology |
|
|
| Infections Infections Invasion of the host organism by microorganisms or their toxins or by parasites that can cause pathological conditions or diseases. Chronic Granulomatous Disease |
|
Anemia is most commonly classified into the subtype microcytic, normocytic, or macrocytic based on the size of the RBCs RBCs Erythrocytes, or red blood cells (RBCs), are the most abundant cells in the blood. While erythrocytes in the fetus are initially produced in the yolk sac then the liver, the bone marrow eventually becomes the main site of production. Erythrocytes: Histology. MCV reflects the size of the RBCs RBCs Erythrocytes, or red blood cells (RBCs), are the most abundant cells in the blood. While erythrocytes in the fetus are initially produced in the yolk sac then the liver, the bone marrow eventually becomes the main site of production. Erythrocytes: Histology and is reported on a CBC.
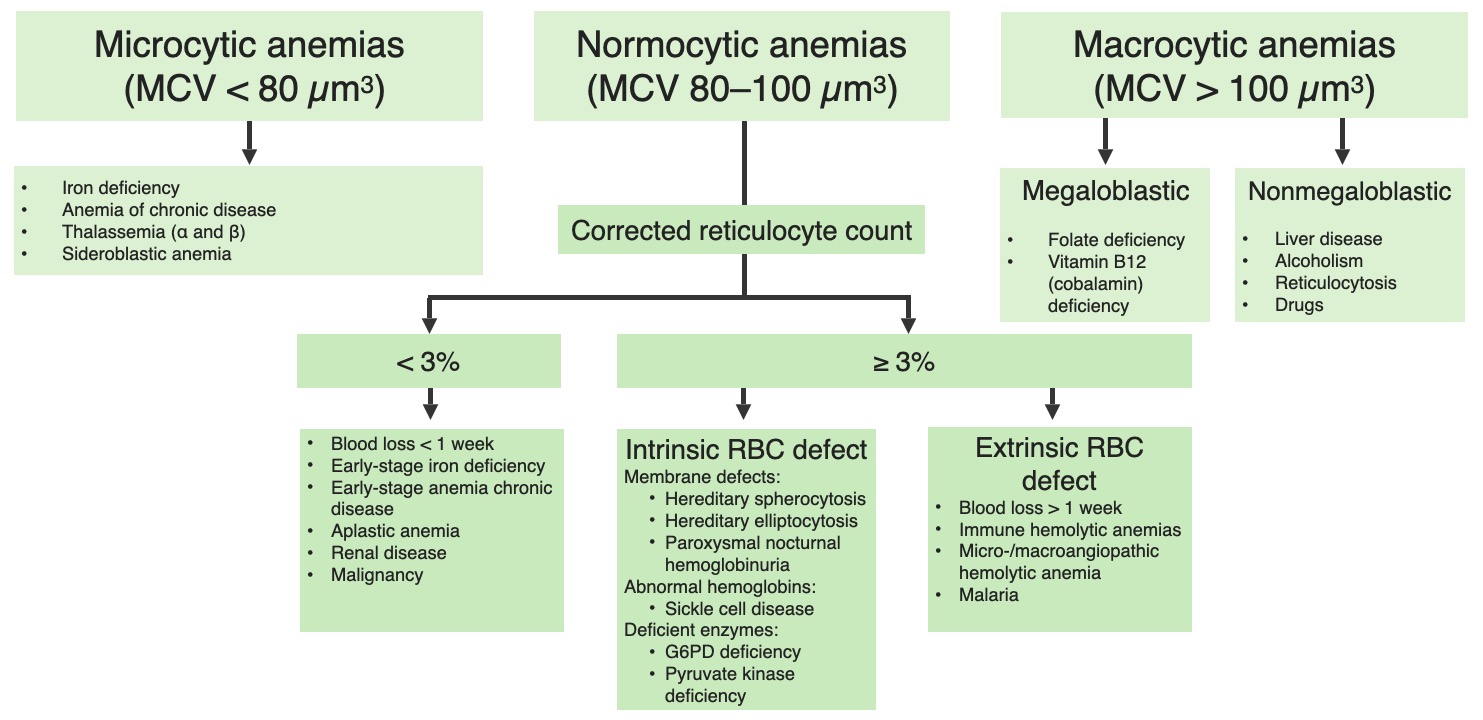
Classification of anemia based on the MCV
Image by Lecturio.Microcytic anemia is characterized by small RBCs RBCs Erythrocytes, or red blood cells (RBCs), are the most abundant cells in the blood. While erythrocytes in the fetus are initially produced in the yolk sac then the liver, the bone marrow eventually becomes the main site of production. Erythrocytes: Histology with a low MCV. These features are usually due to a decreased Hb Hb The oxygen-carrying proteins of erythrocytes. They are found in all vertebrates and some invertebrates. The number of globin subunits in the hemoglobin quaternary structure differs between species. Structures range from monomeric to a variety of multimeric arrangements. Gas Exchange content within the RBCs RBCs Erythrocytes, or red blood cells (RBCs), are the most abundant cells in the blood. While erythrocytes in the fetus are initially produced in the yolk sac then the liver, the bone marrow eventually becomes the main site of production. Erythrocytes: Histology. Causes include:
Mnemonic:
To remember the causes of microcytic anemia use the mnemonic TAILS:
Normocytic anemias have a normal MCV. Often, the reticulocyte Reticulocyte Immature erythrocytes. In humans, these are erythroid cells that have just undergone extrusion of their cell nucleus. They still contain some organelles that gradually decrease in number as the cells mature. Ribosomes are last to disappear. Certain staining techniques cause components of the ribosomes to precipitate into characteristic ‘reticulum’ (not the same as the endoplasmic reticulum), hence the name reticulocytes. Erythrocytes: Histology count is used to help narrow the differential diagnosis. (Reticulocytes are “new” blood cells in circulation Circulation The movement of the blood as it is pumped through the cardiovascular system. ABCDE Assessment, and their level reflects the rate of new RBC production.) Causes of normocytic anemia include:
Hemolytic anemias:
Nonhemolytic anemias:
Macrocytic anemias have an elevated MCV, or large RBCs RBCs Erythrocytes, or red blood cells (RBCs), are the most abundant cells in the blood. While erythrocytes in the fetus are initially produced in the yolk sac then the liver, the bone marrow eventually becomes the main site of production. Erythrocytes: Histology. Causes include:
Important details when considering a diagnosis of anemia:
Symptoms develop based on the rate and severity of Hb Hb The oxygen-carrying proteins of erythrocytes. They are found in all vertebrates and some invertebrates. The number of globin subunits in the hemoglobin quaternary structure differs between species. Structures range from monomeric to a variety of multimeric arrangements. Gas Exchange decrease from baseline. Anemia is also often asymptomatic and found on laboratory studies only.
| Body region/organ system | Symptoms | Possible physical exam findings |
|---|---|---|
| General |
|
|
| Integumentary |
|
|
| Cardiorespiratory |
|
|
| Head, eyes, ears, nose Nose The nose is the human body’s primary organ of smell and functions as part of the upper respiratory system. The nose may be best known for inhaling oxygen and exhaling carbon dioxide, but it also contributes to other important functions, such as tasting. The anatomy of the nose can be divided into the external nose and the nasal cavity. Nose Anatomy (External & Internal), and throat Throat The pharynx is a component of the digestive system that lies posterior to the nasal cavity, oral cavity, and larynx. The pharynx can be divided into the oropharynx, nasopharynx, and laryngopharynx. Pharyngeal muscles play an integral role in vital processes such as breathing, swallowing, and speaking. Pharynx: Anatomy (HEENT) | Epistaxis Epistaxis Bleeding from the nose. Granulomatosis with Polyangiitis |
|
| Neurologic |
|
|
| GI/abdomen |
|
|
| Genitourinary |
|
|
The diagnostic process typically starts by assessing the CBC, MCV, and reticulocyte Reticulocyte Immature erythrocytes. In humans, these are erythroid cells that have just undergone extrusion of their cell nucleus. They still contain some organelles that gradually decrease in number as the cells mature. Ribosomes are last to disappear. Certain staining techniques cause components of the ribosomes to precipitate into characteristic ‘reticulum’ (not the same as the endoplasmic reticulum), hence the name reticulocytes. Erythrocytes: Histology count, which are best evaluated simultaneously to start narrowing the differential diagnosis. Additional studies may be obtained on the basis of the patient’s clinical presentation.
A peripheral blood smear Blood smear Myeloperoxidase Deficiency can be helpfuling in establishing the underlying cause of an anemia.
| Abnormality | Description | Associated pathology |
|---|---|---|
| Sickle cells | Sickle-shaped cells |
|
| Howell-Jolly bodies Howell-Jolly Bodies Asplenia | Peripherally located purple bodies representing nuclear remnants of RBCs RBCs Erythrocytes, or red blood cells (RBCs), are the most abundant cells in the blood. While erythrocytes in the fetus are initially produced in the yolk sac then the liver, the bone marrow eventually becomes the main site of production. Erythrocytes: Histology |
|
| Bite cells (degmacytes) | RBCs RBCs Erythrocytes, or red blood cells (RBCs), are the most abundant cells in the blood. While erythrocytes in the fetus are initially produced in the yolk sac then the liver, the bone marrow eventually becomes the main site of production. Erythrocytes: Histology with loss of semicircular portions | G6PD G6PD Pentose Phosphate Pathway |
| Heinz bodies Heinz bodies Abnormal intracellular inclusions, composed of denatured hemoglobin, found on the membrane of red blood cells. They are seen in thalassemias, enzymopathies, hemoglobinopathies, and after splenectomy. Asplenia | Red-pink inclusions within RBCs RBCs Erythrocytes, or red blood cells (RBCs), are the most abundant cells in the blood. While erythrocytes in the fetus are initially produced in the yolk sac then the liver, the bone marrow eventually becomes the main site of production. Erythrocytes: Histology representing aggregates of abnormal Hb Hb The oxygen-carrying proteins of erythrocytes. They are found in all vertebrates and some invertebrates. The number of globin subunits in the hemoglobin quaternary structure differs between species. Structures range from monomeric to a variety of multimeric arrangements. Gas Exchange |
|
| Spherocytes | Dense, small spherical cells with no central pallor |
|
| Schistocytes Schistocytes Hemolytic Uremic Syndrome | Irregularly shaped RBC fragments | Due to mechanical damage:
|
| Normoblasts | Nucleated RBCs RBCs Erythrocytes, or red blood cells (RBCs), are the most abundant cells in the blood. While erythrocytes in the fetus are initially produced in the yolk sac then the liver, the bone marrow eventually becomes the main site of production. Erythrocytes: Histology | Severe hemolysis |
| Target cells (codocytes) | Bull’s-eye appearance (central area of hemoglobinization) |
|
| Basophilic stippling | Purple-blue dots within RBCs RBCs Erythrocytes, or red blood cells (RBCs), are the most abundant cells in the blood. While erythrocytes in the fetus are initially produced in the yolk sac then the liver, the bone marrow eventually becomes the main site of production. Erythrocytes: Histology representing ribosomal precipitates |
|
| Dacrocytes ( teardrop cells Teardrop Cells Primary Myelofibrosis) | Teardrop-shaped RBCs RBCs Erythrocytes, or red blood cells (RBCs), are the most abundant cells in the blood. While erythrocytes in the fetus are initially produced in the yolk sac then the liver, the bone marrow eventually becomes the main site of production. Erythrocytes: Histology | Due to
bone marrow infiltration
Bone Marrow Infiltration
Multiple Myeloma:
|
| Macroovalocytes | Large, oval-shaped erythrocytes Erythrocytes Erythrocytes, or red blood cells (RBCs), are the most abundant cells in the blood. While erythrocytes in the fetus are initially produced in the yolk sac then the liver, the bone marrow eventually becomes the main site of production. Erythrocytes: Histology | Megaloblastic anemia Megaloblastic anemia Megaloblastic anemia is a subset of macrocytic anemias that arises because of impaired nucleic acid synthesis in erythroid precursors. This impairment leads to ineffective RBC production and intramedullary hemolysis that is characterized by large cells with arrested nuclear maturation. The most common causes are vitamin B12 and folic acid deficiencies. Megaloblastic Anemia |
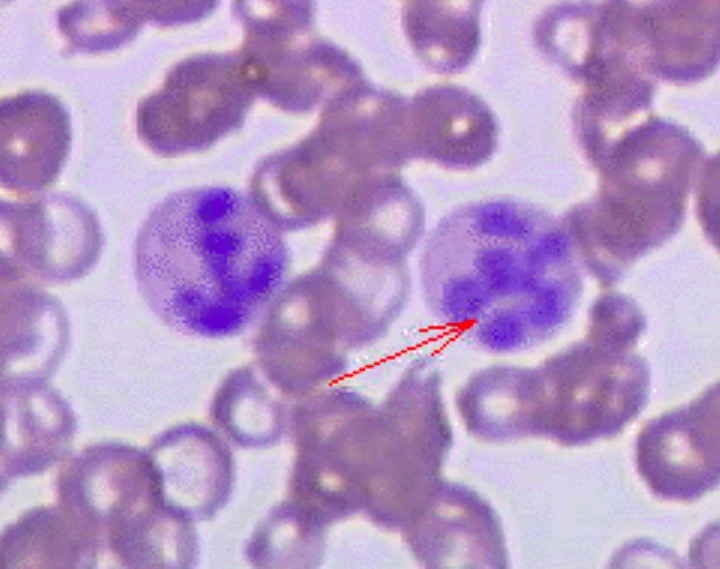
Peripheral blood smear of a patient with megaloblastic anemia:
The red arrow points to a hypersegmented neutrophil.
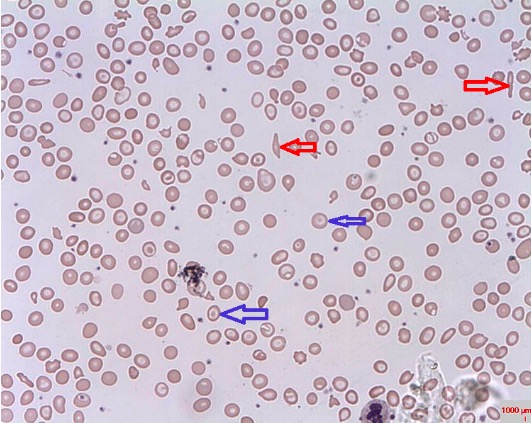
Blood smear displaying sickle cells (red arrows) and target cells (blue arrows)
Image: “Target cells” by Prof. Osaro Erhabor. License: CC0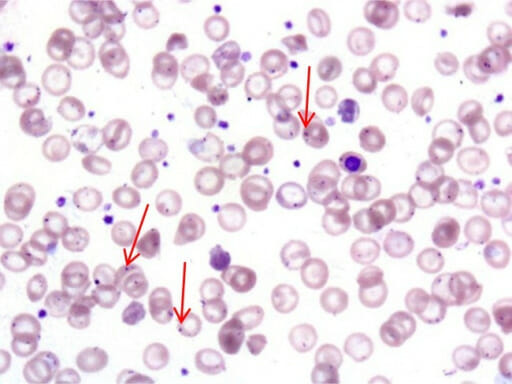
Howell-Jolly bodies (red arrows) in circulating RBCs of an individual with overwhelming postsplenectomy infection (OPSI)
Image: “f2-tm_6p02” by Serio B., Pezzullo L. et al. License: CC BY 2.5
Blood film showing spherocytes
Image: “Spherocytes” by Ed Uthman. License: CC BY 2.0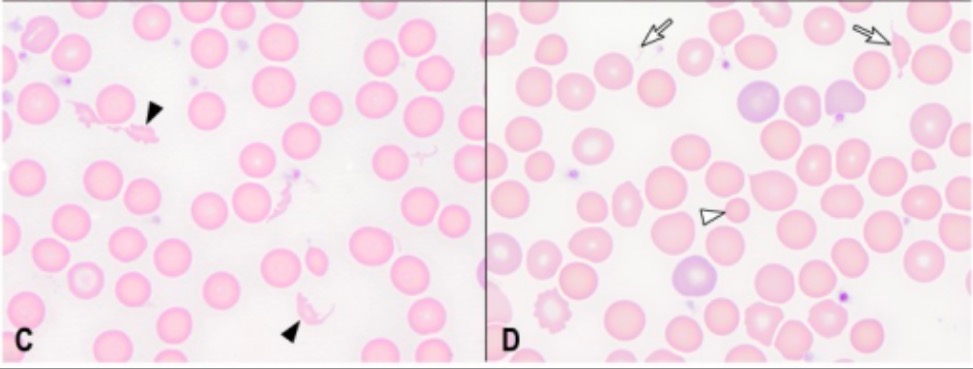
Left: schistocytes (closed arrowheads)
Right: spherocytes (open arrowhead) and schistocytes (open arrows)
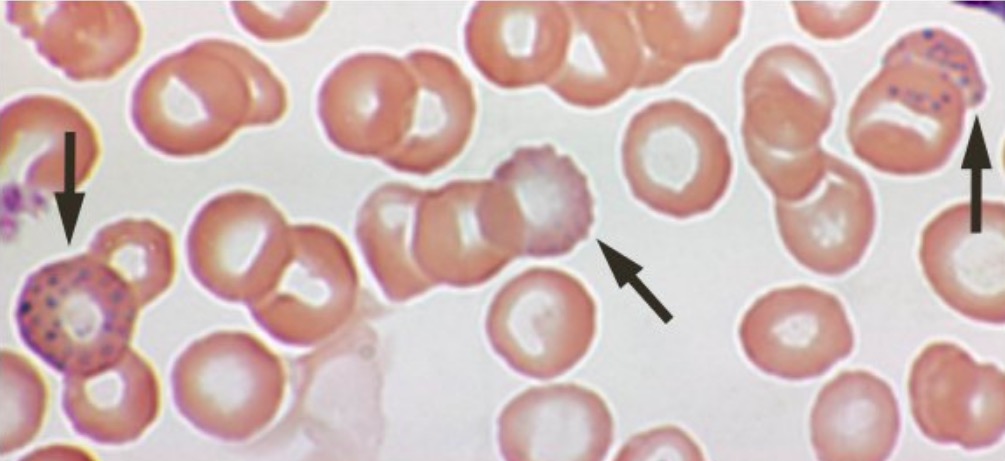
RBC basophilic stippling (arrows)
Image: “Lead poisoning – blood film” by Herbert L. Fred, MD and Hendrik A. van Dijk. License: CC BY 2.0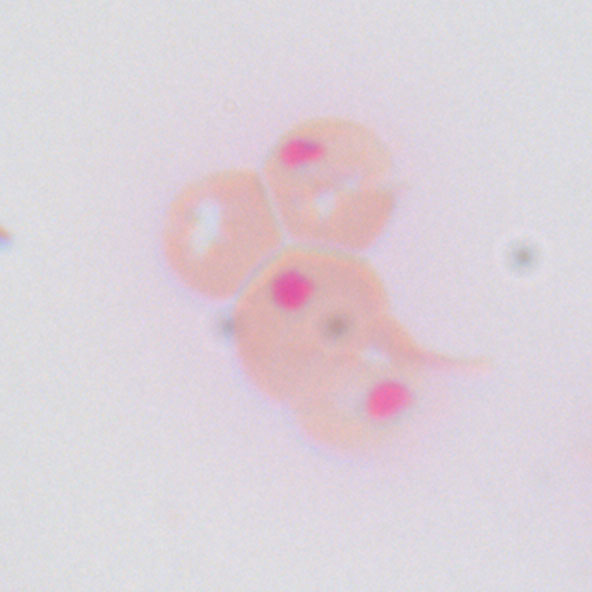
Heinz bodies in peripheral blood (as seen in G6PD deficiency)
Image: “Heinz bodies cat” by Ailuromancy. License: Public DomainIf lifestyle factors are thought to be contributing to the anemia, education can be provided regarding:
Hemolytic anemias are result of the destruction or premature Premature Childbirth before 37 weeks of pregnancy (259 days from the first day of the mother’s last menstrual period, or 245 days after fertilization). Necrotizing Enterocolitis clearance of RBCs RBCs Erythrocytes, or red blood cells (RBCs), are the most abundant cells in the blood. While erythrocytes in the fetus are initially produced in the yolk sac then the liver, the bone marrow eventually becomes the main site of production. Erythrocytes: Histology. Specifically, they can be due to either damage caused by a narrowed vascular lumen ( intravascular hemolysis Intravascular hemolysis Hemolytic Anemia) or increased splenic clearance ( extravascular hemolysis Extravascular hemolysis Hemolytic Anemia). Splenic clearance can result from intrinsic abnormalities of the RBCs RBCs Erythrocytes, or red blood cells (RBCs), are the most abundant cells in the blood. While erythrocytes in the fetus are initially produced in the yolk sac then the liver, the bone marrow eventually becomes the main site of production. Erythrocytes: Histology (e.g., abnormal membranes, enzymes Enzymes Enzymes are complex protein biocatalysts that accelerate chemical reactions without being consumed by them. Due to the body’s constant metabolic needs, the absence of enzymes would make life unsustainable, as reactions would occur too slowly without these molecules. Basics of Enzymes, or Hb Hb The oxygen-carrying proteins of erythrocytes. They are found in all vertebrates and some invertebrates. The number of globin subunits in the hemoglobin quaternary structure differs between species. Structures range from monomeric to a variety of multimeric arrangements. Gas Exchange) or extrinsic antibody coating by the immune system Immune system The body’s defense mechanism against foreign organisms or substances and deviant native cells. It includes the humoral immune response and the cell-mediated response and consists of a complex of interrelated cellular, molecular, and genetic components. Primary Lymphatic Organs (e.g., ABO incompatibility ABO incompatibility Hemolytic Disease of the Fetus and Newborn).
Examples of hemolytic anemias include: