Hemolytic disease of the fetus and newborn Newborn An infant during the first 28 days after birth. Physical Examination of the Newborn (HDFN), also known as erythroblastosis fetalis, is caused by maternal IgG IgG The major immunoglobulin isotype class in normal human serum. There are several isotype subclasses of igg, for example, igg1, igg2a, and igg2b. Hypersensitivity Pneumonitis antibody destruction of the fetal RBCs RBCs Erythrocytes, or red blood cells (RBCs), are the most abundant cells in the blood. While erythrocytes in the fetus are initially produced in the yolk sac then the liver, the bone marrow eventually becomes the main site of production. Erythrocytes: Histology. Rhesus (Rh) blood group incompatibility (frequently triggered by D antigen Antigen Substances that are recognized by the immune system and induce an immune reaction. Vaccination) and ABO incompatibility are common causes. In Rh incompatibility, an RhD-negative mother carries an RhD-positive baby; thus, antibodies Antibodies Immunoglobulins (Igs), also known as antibodies, are glycoprotein molecules produced by plasma cells that act in immune responses by recognizing and binding particular antigens. The various Ig classes are IgG (the most abundant), IgM, IgE, IgD, and IgA, which differ in their biologic features, structure, target specificity, and distribution. Immunoglobulins: Types and Functions form against antigens when fetal RBCs RBCs Erythrocytes, or red blood cells (RBCs), are the most abundant cells in the blood. While erythrocytes in the fetus are initially produced in the yolk sac then the liver, the bone marrow eventually becomes the main site of production. Erythrocytes: Histology cross into the maternal circulation Circulation The movement of the blood as it is pumped through the cardiovascular system. ABCDE Assessment. In ABO incompatibility, commonly, a mother with blood type O has existing antibodies Antibodies Immunoglobulins (Igs), also known as antibodies, are glycoprotein molecules produced by plasma cells that act in immune responses by recognizing and binding particular antigens. The various Ig classes are IgG (the most abundant), IgM, IgE, IgD, and IgA, which differ in their biologic features, structure, target specificity, and distribution. Immunoglobulins: Types and Functions to A and B antigens. The affected baby can suffer from hemolytic anemia Hemolytic Anemia Hemolytic anemia (HA) is the term given to a large group of anemias that are caused by the premature destruction/hemolysis of circulating red blood cells (RBCs). Hemolysis can occur within (intravascular hemolysis) or outside the blood vessels (extravascular hemolysis). Hemolytic Anemia leading to severe neonatal jaundice Neonatal jaundice Yellow discoloration of the skin; mucous membrane; and sclera in the newborn. It is a sign of neonatal hyperbilirubinemia. Most cases are transient self-limiting (physiological neonatal jaundice) occurring in the first week of life, but some can be a sign of pathological disorders, particularly liver diseases. Jaundice, hydrops Hydrops Cholecystitis fetalis, cardiac complications, and fetal demise. If the pregnancy Pregnancy The status during which female mammals carry their developing young (embryos or fetuses) in utero before birth, beginning from fertilization to birth. Pregnancy: Diagnosis, Physiology, and Care is affected by Rh incompatibility, antenatal surveillance Surveillance Developmental Milestones and Normal Growth is done to determine the need for intrauterine transfusion and early delivery. Postnatal treatment includes close monitoring, phototherapy Phototherapy Treatment of disease by exposure to light, especially by variously concentrated light rays or specific wavelengths. Hyperbilirubinemia of the Newborn for jaundice Jaundice Jaundice is the abnormal yellowing of the skin and/or sclera caused by the accumulation of bilirubin. Hyperbilirubinemia is caused by either an increase in bilirubin production or a decrease in the hepatic uptake, conjugation, or excretion of bilirubin. Jaundice, and exchange transfusion in severe cases. For RhD-negative mothers, maternal sensitization can be prevented by using anti-D immunoglobulin (RhoGAM). Prognosis Prognosis A prediction of the probable outcome of a disease based on a individual's condition and the usual course of the disease as seen in similar situations. Non-Hodgkin Lymphomas is excellent with prenatal care Prenatal care Prenatal care is a systematic and periodic assessment of pregnant women during gestation to assure the best health outcome for the mother and her fetus. Prenatal care prevents and identifies maternal and fetal problems that adversely affect the pregnancy outcome. Prenatal Care, blood type screening Screening Preoperative Care, and availability of RhD immune globulin.
Last updated: Dec 22, 2022
Hemolytic disease of the fetus and newborn Newborn An infant during the first 28 days after birth. Physical Examination of the Newborn (HDFN) is hemolytic anemia Hemolytic Anemia Hemolytic anemia (HA) is the term given to a large group of anemias that are caused by the premature destruction/hemolysis of circulating red blood cells (RBCs). Hemolysis can occur within (intravascular hemolysis) or outside the blood vessels (extravascular hemolysis). Hemolytic Anemia of the fetus or newborn Newborn An infant during the first 28 days after birth. Physical Examination of the Newborn of varying degrees of severity due to maternal IgG IgG The major immunoglobulin isotype class in normal human serum. There are several isotype subclasses of igg, for example, igg1, igg2a, and igg2b. Hypersensitivity Pneumonitis antibodies Antibodies Immunoglobulins (Igs), also known as antibodies, are glycoprotein molecules produced by plasma cells that act in immune responses by recognizing and binding particular antigens. The various Ig classes are IgG (the most abundant), IgM, IgE, IgD, and IgA, which differ in their biologic features, structure, target specificity, and distribution. Immunoglobulins: Types and Functions against fetal RBC surface antigens.
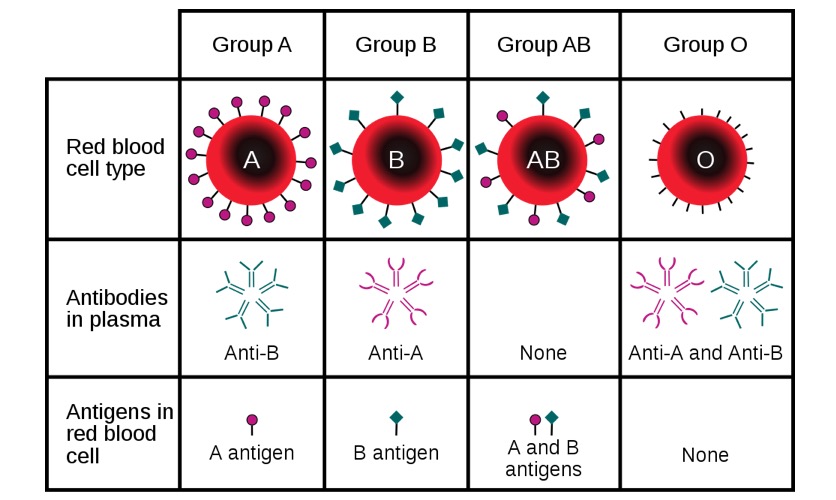
Major ABO blood groups with the respective antigens and antibodies based on blood type
Image: “ABO blood type” by InvictaHOG. License: Public DomainDuring pregnancy Pregnancy The status during which female mammals carry their developing young (embryos or fetuses) in utero before birth, beginning from fertilization to birth. Pregnancy: Diagnosis, Physiology, and Care, fetal RBCs RBCs Erythrocytes, or red blood cells (RBCs), are the most abundant cells in the blood. While erythrocytes in the fetus are initially produced in the yolk sac then the liver, the bone marrow eventually becomes the main site of production. Erythrocytes: Histology move across the placenta Placenta A highly vascularized mammalian fetal-maternal organ and major site of transport of oxygen, nutrients, and fetal waste products. It includes a fetal portion (chorionic villi) derived from trophoblasts and a maternal portion (decidua) derived from the uterine endometrium. The placenta produces an array of steroid, protein and peptide hormones (placental hormones). Placenta, Umbilical Cord, and Amniotic Cavity into the maternal circulation Circulation The movement of the blood as it is pumped through the cardiovascular system. ABCDE Assessment:
ABO incompatibility:
Rhesus incompatibility:
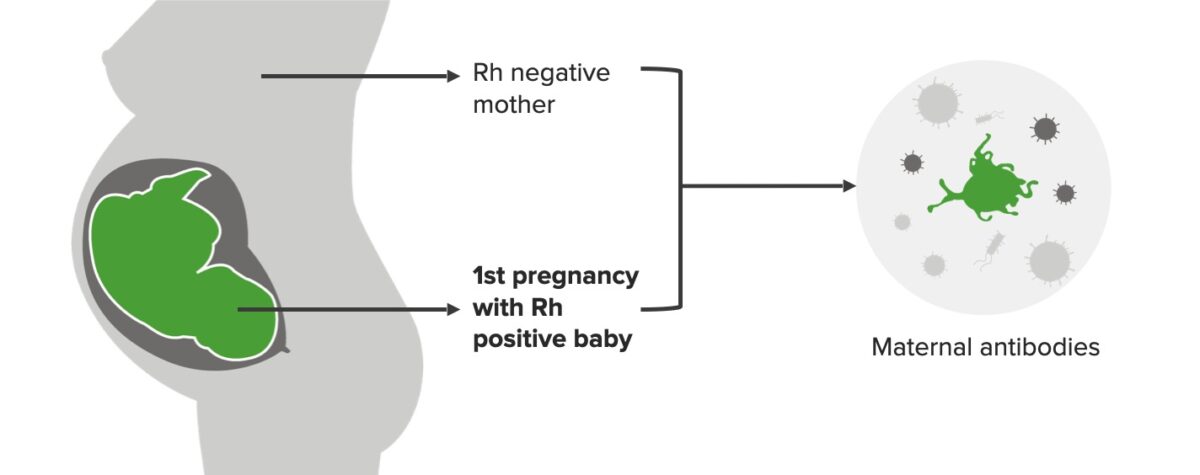
Rh incompatibility, 1st pregnancy:
Mother is Rh negative and baby is Rh positive in the 1st pregnancy, which triggers the formation of maternal antibodies (IgM). This scenario does not affect the 1st baby.
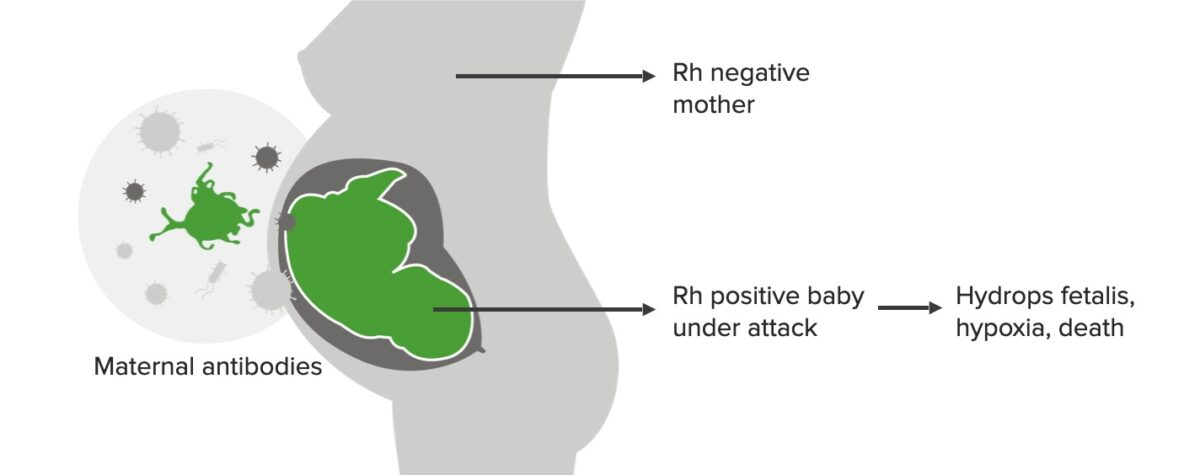
Rh incompatibility, 2nd pregnancy:
While the 1st-born baby is not affected, by this time, IgG maternal antibodies have developed, which attack the baby if Rh positive. This scenario can lead to hydrops fetalis, hypoxia, and death.
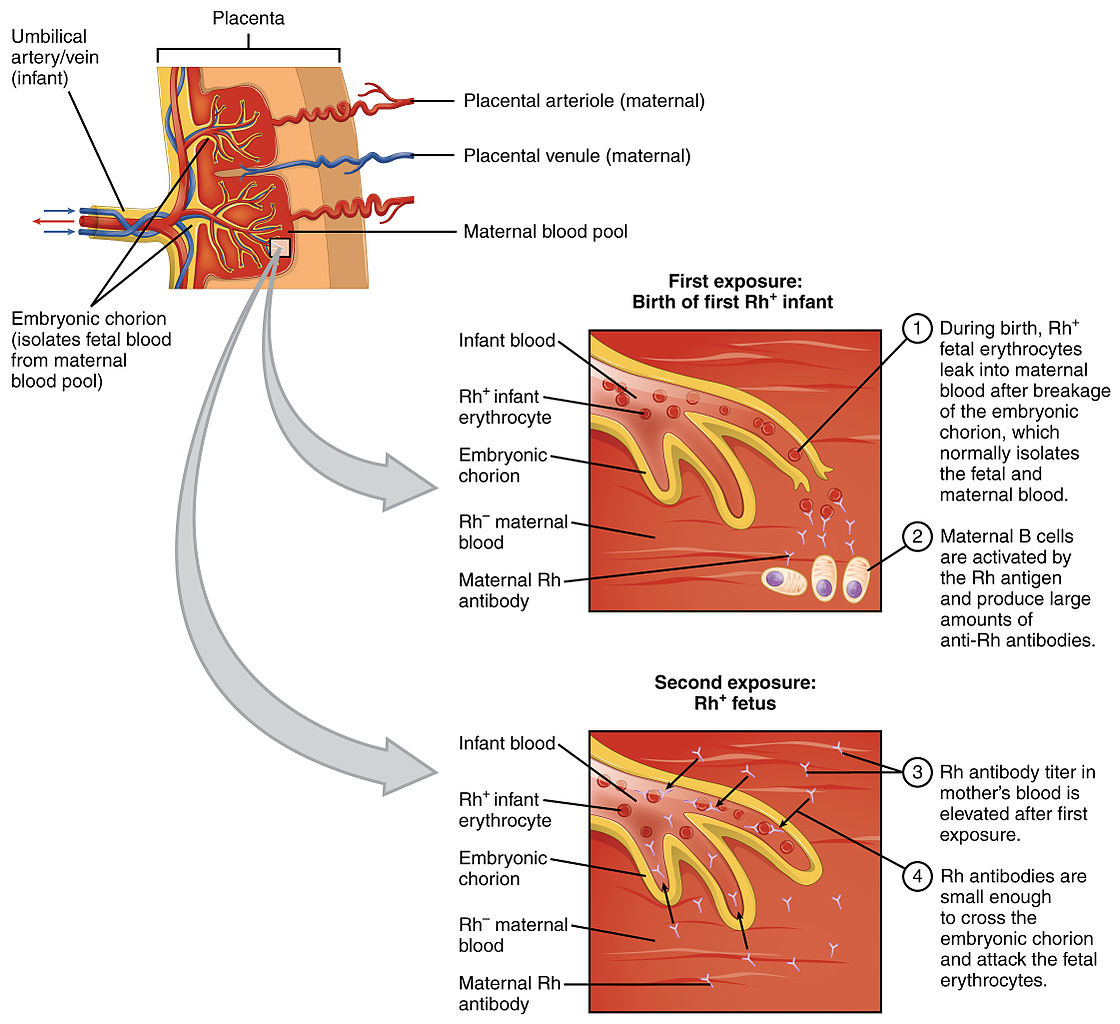
Pathophysiology of Rhesus incompatibility during pregnancies
Image: “Erythroblastosis Fetalis” by OpenStax College. License: CC BY 3.0Ultrasound (US) may show evidence of immune hydrops Hydrops Cholecystitis fetalis, a life-threatening condition in which fetuses have abnormal fluid build-up in the body. The US findings of immune hydrops Hydrops Cholecystitis fetalis may include:
Mild-to-moderate disease:
Severe disease:
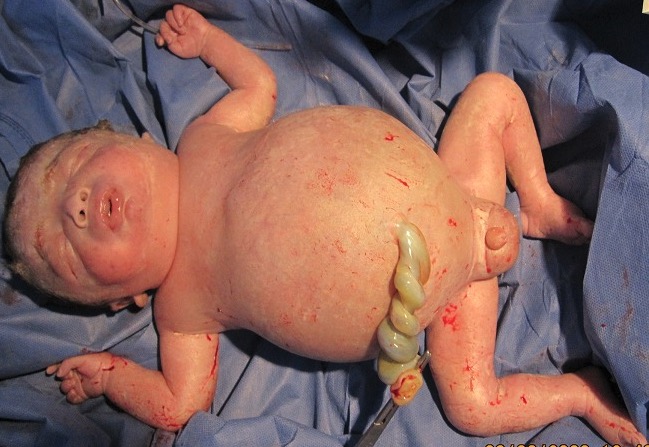
Hydrops fetalis in a newborn, born to a Rhesus negative mother without proper prevention:
Note the generalized edema.
It is important to identify the potential risk factors for unknown sensitization and prior history of HDFN during pregnancy Pregnancy The status during which female mammals carry their developing young (embryos or fetuses) in utero before birth, beginning from fertilization to birth. Pregnancy: Diagnosis, Physiology, and Care. Potential blood incompatibilities include:
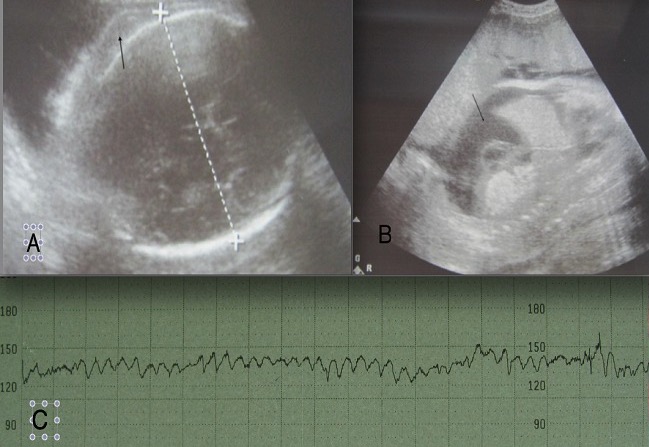
Diagnosis of hemolytic disease of the fetus and newborn
A: ultrasound image of the head of the fetus showing scalp edema (arrow); B: ultrasound showing ascites (arrow) on a sagittal section of the abdomen; C: sinusoidal fetal heart-rate pattern seen in patients with severe anemia
RhD incompatibilities are the only forms of alloimmunization that can be prevented.
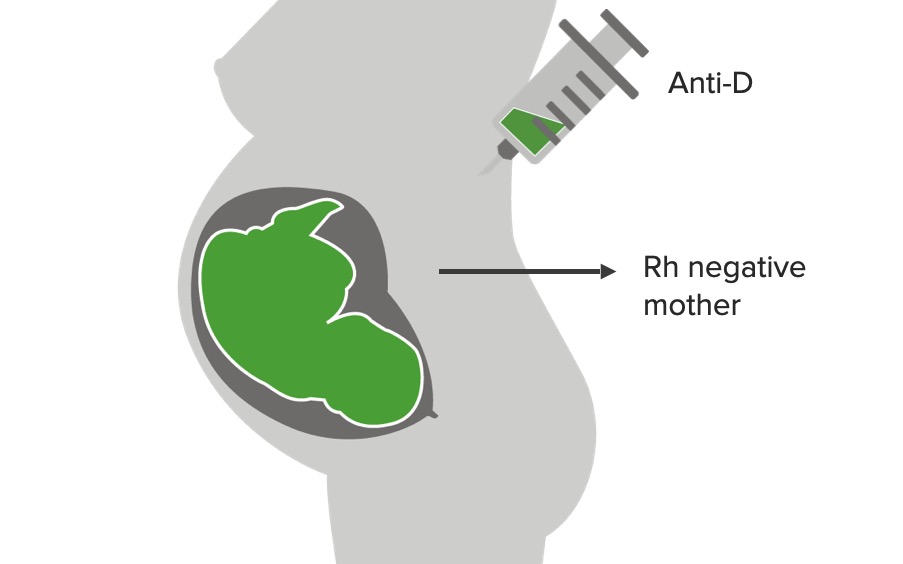
Prevention of sensitization using anti-D immunoglobulins (RhoGAM):
Anti-D binds Rh negative antigens in the mother’s circulation to avoid sensitization and development of immune response/formation of antibodies versus Rh negative.
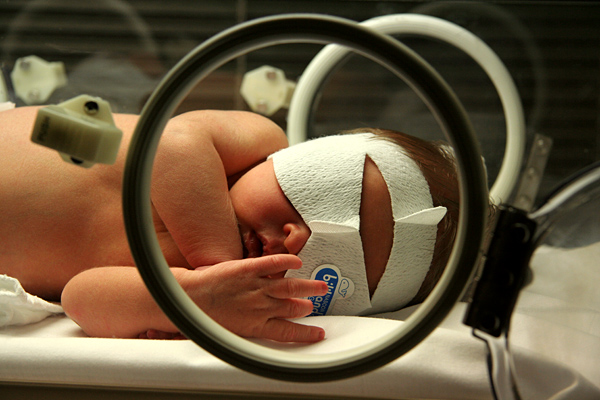
Image of neonatal jaundice: newborn undergoing phototherapy
Image: “Jaundice phototherapy” by Martin Pot. License: CC BY 3.0