The most common benign Benign Fibroadenoma liver Liver The liver is the largest gland in the human body. The liver is found in the superior right quadrant of the abdomen and weighs approximately 1.5 kilograms. Its main functions are detoxification, metabolism, nutrient storage (e.g., iron and vitamins), synthesis of coagulation factors, formation of bile, filtration, and storage of blood. Liver: Anatomy tumors include hepatic hemangiomas, focal nodular hyperplasia Hyperplasia An increase in the number of cells in a tissue or organ without tumor formation. It differs from hypertrophy, which is an increase in bulk without an increase in the number of cells. Cellular Adaptation, and hepatic adenomas. These tumors are mostly asymptomatic and/or found incidentally on abdominal imaging. While these tumors are benign Benign Fibroadenoma, large lesions can cause symptoms such as upper abdominal pain Abdominal Pain Acute Abdomen, or produce complications such as bleeding. Malignant potential is a concern for hepatic adenoma, depending on risk factors. The diagnosis is based on imaging studies, with characteristic findings defining the tumor Tumor Inflammation. Biopsy Biopsy Removal and pathologic examination of specimens from the living body. Ewing Sarcoma generally is reserved for equivocal cases. Management is observation for most small, asymptomatic, and non-growing tumors. However, high-risk factors, symptoms, increasing tumor Tumor Inflammation size, and complications dictate the need for surgical intervention.
Last updated: Jan 2, 2024
| Hepatic hemangioma Hemangioma A vascular anomaly due to proliferation of blood vessels that forms a tumor-like mass. The common types involve capillaries and veins. It can occur anywhere in the body but is most frequently noticed in the skin and subcutaneous tissue. Imaging of the Liver and Biliary Tract | Focal nodular hyperplasia Hyperplasia An increase in the number of cells in a tissue or organ without tumor formation. It differs from hypertrophy, which is an increase in bulk without an increase in the number of cells. Cellular Adaptation | Hepatocellular adenoma | Hepatocellular carcinoma Hepatocellular carcinoma Hepatocellular carcinoma (HCC) typically arises in a chronically diseased or cirrhotic liver and is the most common primary liver cancer. Diagnosis may include ultrasound, CT, MRI, biopsy (if inconclusive imaging), and/or biomarkers. Hepatocellular Carcinoma (HCC) and Liver Metastases | |
|---|---|---|---|---|
| Characteristics and pathologic features | Cavernous vascular spaces | Central stellate scar Scar Dermatologic Examination + portal tracts, bile ductules Bile ductules Gallbladder and Biliary Tract: Anatomy, Kupffer cells | Sheets of enlarged hepatocytes Hepatocytes The main structural component of the liver. They are specialized epithelial cells that are organized into interconnected plates called lobules. Liver: Anatomy; no portal tracts or bile ductules Bile ductules Gallbladder and Biliary Tract: Anatomy | Well-differentiated (similar to normal hepatocytes Hepatocytes The main structural component of the liver. They are specialized epithelial cells that are organized into interconnected plates called lobules. Liver: Anatomy); poorly differentiated (marked cytologic atypia Atypia Fibrocystic Change) |
| Predominant gender Gender Gender Dysphoria affected | Women | Women | Women | Men |
| Clinical history | Oral contraceptive pills (OCPs) may affect growth. | OCP effect not proven; little or no effect on development or growth | OCPs, anabolic steroids a factor | History of cirrhosis Cirrhosis Cirrhosis is a late stage of hepatic parenchymal necrosis and scarring (fibrosis) most commonly due to hepatitis C infection and alcoholic liver disease. Patients may present with jaundice, ascites, and hepatosplenomegaly. Cirrhosis can also cause complications such as hepatic encephalopathy, portal hypertension, portal vein thrombosis, and hepatorenal syndrome. Cirrhosis and risk factors (e.g., hepatitis B Hepatitis B Hepatitis B virus (HBV) is a partially double-stranded DNA virus, which belongs to the Orthohepadnavirus genus and the Hepadnaviridae family. Most individuals with acute HBV infection are asymptomatic or have mild, self-limiting symptoms. Chronic infection can be asymptomatic or create hepatic inflammation, leading to liver cirrhosis and hepatocellular carcinoma (HCC). Hepatitis B Virus) |
| Malignancy Malignancy Hemothorax potential | None | None | Yes | N/A |
| MRI (contrast): arterial phase | Peripheral nodular enhancement | Early homogeneous Homogeneous Imaging of the Spleen/diffuse enhancement | Well-demarcated enhancement; heterogeneous (due to hemorrhage, necrosis Necrosis The death of cells in an organ or tissue due to disease, injury or failure of the blood supply. Ischemic Cell Damage, steatosis Steatosis Metabolic Dysfunction-associated Steatotic Liver Disease (MASLD)) | Hyperenhancement |
| MRI (contrast): venous phase | Progressive centripetal fill-in | Isointense with central scar Scar Dermatologic Examination enhancement |
Variable
Variable
Variables represent information about something that can change. The design of the measurement scales, or of the methods for obtaining information, will determine the data gathered and the characteristics of that data. As a result, a variable can be qualitative or quantitative, and may be further classified into subgroups.
Types of Variables
late phase
Late Phase
Sepsis in Children
|
Portal venous washout |
| Additional key points |
|
60%–70% positive uptake in sulfur colloid Colloid Colloid solutions include large proteins or cells that do not readily cross capillary membranes. They remain in the ecf and do not distribute into the icf (similar to crystalloids). Intravenous Fluids scan | No MRI-specific pattern was identified for β-catenin–mutated HCA. | Rim enhancement on delayed post-contrast images causing a capsule-appearance: relatively specific for HCC HCC Hepatocellular carcinoma (HCC) typically arises in a chronically diseased or cirrhotic liver and is the most common primary liver cancer. Diagnosis may include ultrasound, CT, MRI, biopsy (if inconclusive imaging), and/or biomarkers. Hepatocellular Carcinoma (HCC) and Liver Metastases |
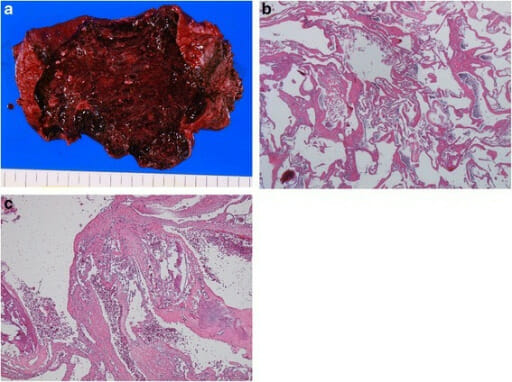
Gross and histological findings of hepatic hemangioma:
a: wine-colored, elastic tumor
b: tumor composed of blood-filled spaces lined by a single layer of endothelial cells without smooth muscle (H&E stain, 40x objective)
c: fibrotic, hyalinized, calcified lesions (H&E stain, 40x objective)
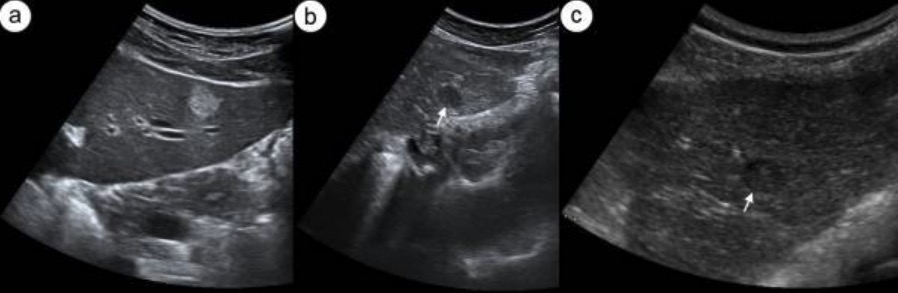
Hepatic hemangioma on ultrasound: Transverse sonogram of left lobe of liver presents typical hyperechogenic hemangioma (a). Figures (b) and (c) show 2 different hypoechogenic liver lesions suspected to be atypical hemangioma (white arrow).
Image: “Hepatic hemangioma in US” by Department of Radiology, Medical University of Gdansk, Debinki 7, 80-211 Gdansk, Poland. License: CC BY 2.0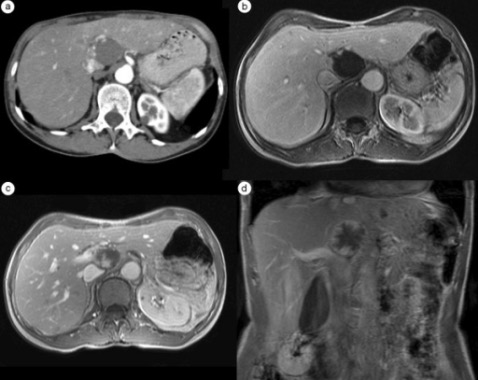
Computed tomography and MRI images of hepatic hemangioma: Hepatic arterial phase (HAP)-CT image visualizes globular type of enhancement (a). Globular pattern of enhancement is also visible in HAP-MR image (b). Progressive fill-in enhancement pattern can be observed in the portal-venous (c) and equilibrium (d) phases of the MR study.
Image: “F4” by Department of Radiology, Medical University of Gdansk, Debinki 7, 80-211 Gdansk, Poland. License: CC BY 2.0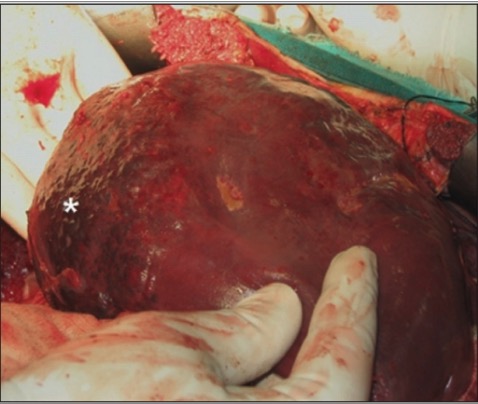
A giant hepatic hemangioma
Image: “F0004” by Department of Gastro-intestinal Surgery, All India Institute of Medical Sciences, Delhi, India. License: CC BY 2.0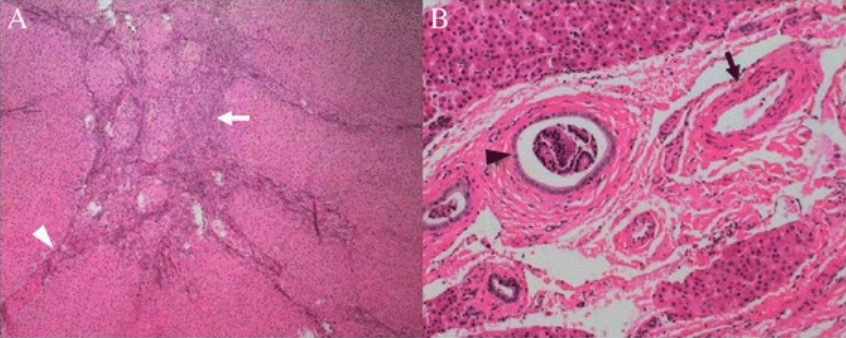
Histologic features of classic FNH: Tumor was subdivided into nodules by fibrous septa (A, white arrowhead) originating from a central scar (A, white arrow). Malformed arteries (B, black arrow) and bile ductular proliferation are demonstrated (B, black arrowhead).
Image: “Histological features of the classic focal nodular hyperplasia” by Department of Radiology, PLA General Hospital, #28 Fuxing Road, Beijing, 100853, China. License: CC BY 4.0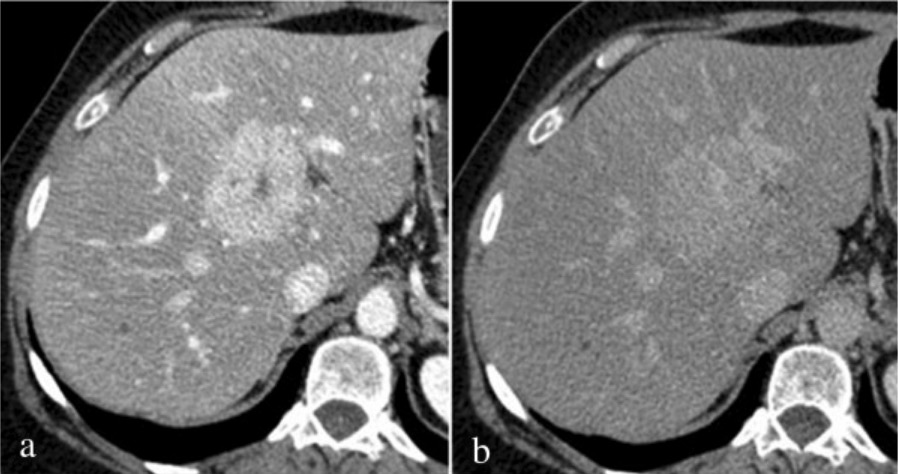
Computed tomography findings of FNH: Mixed phase (hepatic arterial phase/portal venous phase during hepatic enhancement) shows intense homogeneous enhancement with hypodense focal central scar (a); on delayed phase (b), the lesion appears substantially isodense to liver parenchyma with persistent enhancement of central scar.
Image: “Fig3” by Department of Surgical and Biomedical Sciences, Division of Radiology 2, Perugia University, S, Maria della Misericordia Hospital, S, Andrea delle Fratte, 06134 Perugia, Italy. License: CC BY 4.0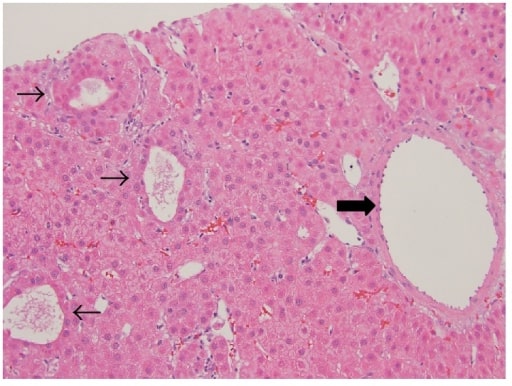
Hepatic adenoma: well-differentiated neoplasm composed of normal-appearing hepatocytes arranged in sheets and thin cords with patchy pseudoacinar growth pattern (thin arrows), scattered inflammatory foci, and bands of fibrosis with unpaired large arteries (thick arrow)
Image: “Hepatic adenoma” by M. I. Montenovo. License: CC BY 4.0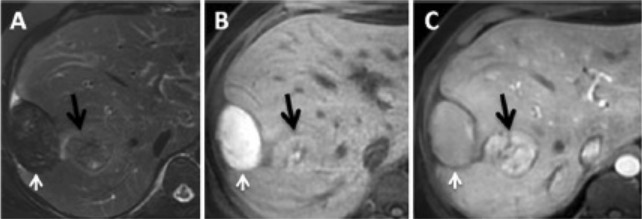
Magnetic resonance imaging in a patient with adenoma with bleeding: white arrow, hematoma; black arrow, hepatic adenoma.
A: T2 sequence with fat saturation shows a hepatic subcapsular nodule with hyposignal.
B: Pre-contrast T1 sequence shows a lesion with hypersignal, signifying products of hemoglobin degradation.
C: Post-contrast T1 sequence in the arterial phase emphasizes the hepatic lesion.