Intravenous fluids (IVFs) are one of the most common interventions administered in medicine to approximate physiologic bodily fluids. Intravenous fluids are divided into 2 categories: crystalloid and colloid solutions. Intravenous fluids have a wide variety of indications, including intravascular volume expansion, electrolyte manipulation, and maintenance fluids. Crystalloids and colloids have different general compositions, which affect distributions through the body’s fluid compartments and guide clinical use. Crystalloid solutions Crystalloid Solutions Isotonic solutions of mineral salts, such as ringer's lactate and sodium chloride (saline solution), used in fluid therapy to rehydrate blood volume. Sepsis in Children are typically used for patients Patients Individuals participating in the health care system for the purpose of receiving therapeutic, diagnostic, or preventive procedures. Clinician–Patient Relationship who are hypovolemic, dehydrated, or have ongoing fluid losses. Colloidal solutions may be used in cases of low oncotic pressure Oncotic Pressure Edema. Providers should choose fluid types based on the clinical scenario and best available evidence. All recipients of IVFs should be closely monitored to determine the goal and status of the fluid therapy.
Last updated: Mar 15, 2023
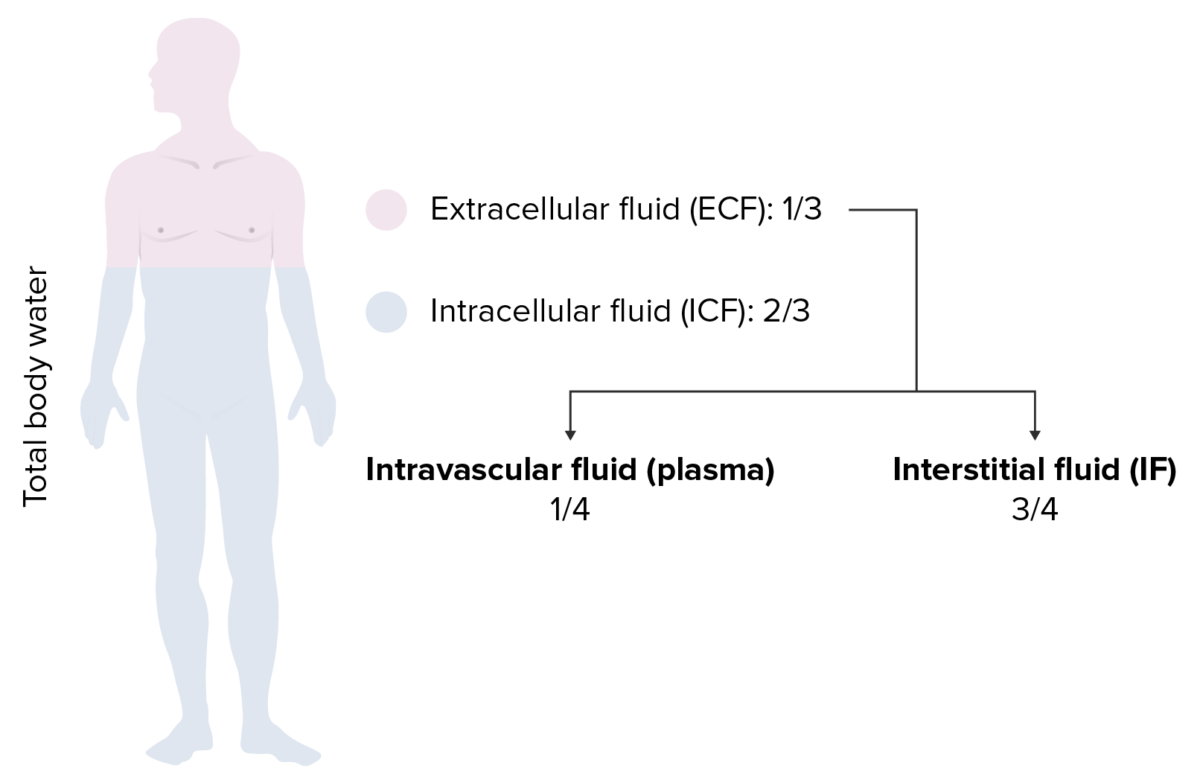
Distribution of total body water (TBW):
⅔ of the body’s water is intracellular fluid (ICF) and ⅓ is extracellular fluid (ECF). Of the ECF, ¾ is interstitial fluid and only ¼ is intravascular fluid.
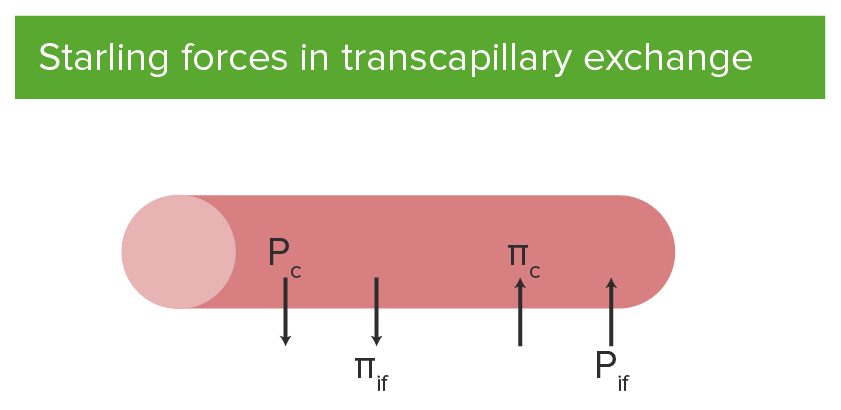
Starling forces in transcapillary exchange:
Outward forces include hydrostatic pressure of blood in the capillary (Pc) and interstitial fluid colloid osmotic pressure (πif).
Inward forces include hydrostatic pressure of the interstitial fluid (Pif) and plasma colloid osmotic pressure (πc) of the capillary.
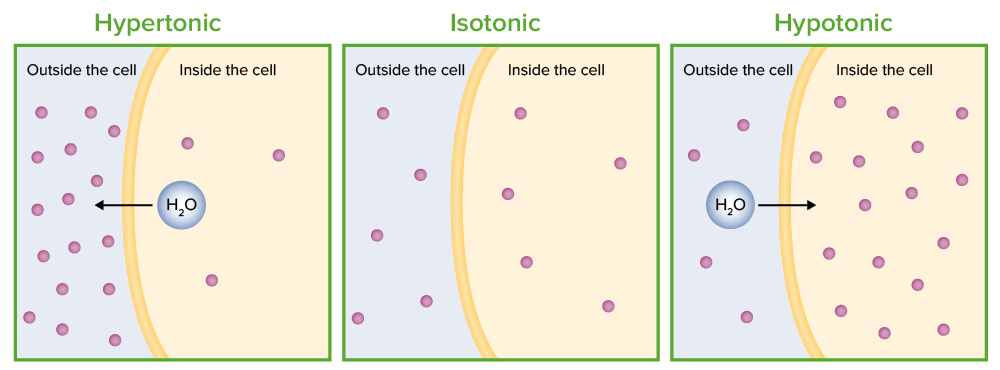
Tonicity: the movement of water due to osmosis of a cell (shown in 3 different solutions):
In the hypertonic solution, water moves out of the cell. In the isotonic solution, there is no net movement of water. In the hypotonic solution, water moves into the cell.
| Fluid | Na mEq/L | Cl mEq/L | K mEq/L | Ca CA Condylomata acuminata are a clinical manifestation of genital HPV infection. Condylomata acuminata are described as raised, pearly, flesh-colored, papular, cauliflower-like lesions seen in the anogenital region that may cause itching, pain, or bleeding. Condylomata Acuminata (Genital Warts)2 mEq/L | Glucose Glucose A primary source of energy for living organisms. It is naturally occurring and is found in fruits and other parts of plants in its free state. It is used therapeutically in fluid and nutrient replacement. Lactose Intolerance g/L | Buffer mEq/L | Osmolarity Osmolarity The concentration of osmotically active particles in solution expressed in terms of osmoles of solute per liter of solution. Osmolality is expressed in terms of osmoles of solute per kilogram of solvent. Hypernatremia mOsm/L | Tonicity Tonicity Plasma tonicity refers to the concentration of only the osmotically active solutes in blood Renal Sodium and Water Regulation |
|---|---|---|---|---|---|---|---|---|
| Normal plasma Plasma The residual portion of blood that is left after removal of blood cells by centrifugation without prior blood coagulation. Transfusion Products | 140 | 100 | 4 | 2.4 | 0.85 | HCO3 24 | 290 | N/A |
| 0.9% saline | 154 | 154 | 0 | 0 | 0 | 0 | 308 | Isotonic Isotonic Solutions having the same osmotic pressure as blood serum, or another solution with which they are compared. Renal Sodium and Water Regulation |
| 0.45% saline | 77 | 77 | 0 | 0 | 0 | 0 | 154 | Hypotonic Hypotonic Solutions that have a lesser osmotic pressure than a reference solution such as blood, plasma, or interstitial fluid. Renal Sodium and Water Regulation |
| Ringer’s lactate | 130 | 109 | 4 | 3 | 0 | Lactate 28 | 273 | Isotonic Isotonic Solutions having the same osmotic pressure as blood serum, or another solution with which they are compared. Renal Sodium and Water Regulation |
| 5% dextrose in water | 0 | 0 | 0 | 0 | 50 | 0 | 252 | Hypotonic Hypotonic Solutions that have a lesser osmotic pressure than a reference solution such as blood, plasma, or interstitial fluid. Renal Sodium and Water Regulation |
| Crystalloids | Colloids | |
|---|---|---|
| Advantages | Cheap | Longer half-life Half-Life The time it takes for a substance (drug, radioactive nuclide, or other) to lose half of its pharmacologic, physiologic, or radiologic activity. Pharmacokinetics and Pharmacodynamics |
| Accessible | A smaller volume is required to expand intravascular volume. | |
| Disadvantages | Shorter half-life Half-Life The time it takes for a substance (drug, radioactive nuclide, or other) to lose half of its pharmacologic, physiologic, or radiologic activity. Pharmacokinetics and Pharmacodynamics | Expensive |
| A larger volume is required to expand intravascular volume. | Risk of allergic reaction |
HCO3 may be indicated for:
Albumin Albumin Serum albumin from humans. It is an essential carrier of both endogenous substances, such as fatty acids and bilirubin, and of xenobiotics in the blood. Liver Function Tests is used for specific purposes:
Frequently reassess goals of IVF therapy and tailor management to avoid adverse effects:
Acid-base abnormalities:
Hypernatremia Hypernatremia Hypernatremia is an elevated serum sodium concentration > 145 mmol/L. Serum sodium is the greatest contributor to plasma osmolality, which is very tightly controlled by the hypothalamus via the thirst mechanism and antidiuretic hormone (ADH) release. Hypernatremia occurs either from a lack of access to water or an excessive intake of sodium. Hypernatremia can result from:
Hyponatremia Hyponatremia Hyponatremia is defined as a decreased serum sodium (sNa+) concentration less than 135 mmol/L. Serum sodium is the greatest contributor to plasma osmolality, which is very tightly controlled via antidiuretic hormone (ADH) release from the hypothalamus and by the thirst mechanism. Hyponatremia:
Hyperkalemia Hyperkalemia Hyperkalemia is defined as a serum potassium (K+) concentration >5.2 mEq/L. Homeostatic mechanisms maintain the serum K+ concentration between 3.5 and 5.2 mEq/L, despite marked variation in dietary intake. Hyperkalemia can be due to a variety of causes, which include transcellular shifts, tissue breakdown, inadequate renal excretion, and drugs. Hyperkalemia:
Hyperglycemia Hyperglycemia Abnormally high blood glucose level. Diabetes Mellitus: