Potassium-sparing diuretics Diuretics Agents that promote the excretion of urine through their effects on kidney function. Heart Failure and Angina Medication are medications that act in the principal cells Principal cells Tubular System in the collecting ducts to induce diuresis that does not result in excretion of potassium Potassium An element in the alkali group of metals with an atomic symbol k, atomic number 19, and atomic weight 39. 10. It is the chief cation in the intracellular fluid of muscle and other cells. Potassium ion is a strong electrolyte that plays a significant role in the regulation of fluid volume and maintenance of the water-electrolyte balance. Hyperkalemia. These diuretics Diuretics Agents that promote the excretion of urine through their effects on kidney function. Heart Failure and Angina Medication include 2 subclasses: sodium channel blockers Sodium Channel Blockers A class of drugs that act by inhibition of sodium influx through cell membranes. Blockade of sodium channels slows the rate and amplitude of initial rapid depolarization, reduces cell excitability, and reduces conduction velocity. Myotonic Dystrophies and aldosterone antagonists Aldosterone antagonists Drugs that bind to and block the activation of mineralocorticoid receptors by mineralocorticoids such as aldosterone. Heart Failure and Angina Medication. Because these agents act so distally in the nephron Nephron The functional units of the kidney, consisting of the glomerulus and the attached tubule. Kidneys: Anatomy, there is no significant potassium Potassium An element in the alkali group of metals with an atomic symbol k, atomic number 19, and atomic weight 39. 10. It is the chief cation in the intracellular fluid of muscle and other cells. Potassium ion is a strong electrolyte that plays a significant role in the regulation of fluid volume and maintenance of the water-electrolyte balance. Hyperkalemia wasting associated with their use. These agents are typically used in the treatment of mineralocorticoid excess Mineralocorticoid excess A hereditary disease characterized by childhood onset hypertension, hypokalemic alkalosis, and low renin and aldosterone secretion. It results from a defect in the activity of the 11-beta-hydroxysteroid dehydrogenase type 2 enzyme which results in inadequate conversion of cortisol to cortisone. The build up of unprocessed cortisol to levels that stimulate mineralocorticoid receptors creates the appearance of having excessive mineralocorticoids. Metabolic Alkalosis, which may be either primary (e.g., Conn syndrome Conn Syndrome Hyperaldosteronism) or secondary (e.g., states of depleted intravascular volume, especially in heart failure Heart Failure A heterogeneous condition in which the heart is unable to pump out sufficient blood to meet the metabolic need of the body. Heart failure can be caused by structural defects, functional abnormalities (ventricular dysfunction), or a sudden overload beyond its capacity. Chronic heart failure is more common than acute heart failure which results from sudden insult to cardiac function, such as myocardial infarction. Total Anomalous Pulmonary Venous Return (TAPVR) patients Patients Individuals participating in the health care system for the purpose of receiving therapeutic, diagnostic, or preventive procedures. Clinician–Patient Relationship). Additionally, spironolactone can be used for its antiandrogenic properties in women with androgen excess (e.g., hirsutism Hirsutism A condition observed in women and children when there is excess coarse body hair of an adult male distribution pattern, such as facial and chest areas. It is the result of elevated androgens from the ovaries, the adrenal glands, or exogenous sources. The concept does not include hypertrichosis, which is an androgen-independent excessive hair growth. Polycystic Ovarian Syndrome) and for androgen suppression Suppression Defense Mechanisms in transgender Transgender Persons having a sense of persistent identification with, and expression of, gender-coded behaviors not typically associated with one's anatomical sex at birth, with or without a desire to undergo sex reassignment procedures. Gender Dysphoria women (male-to-female). These drugs are contraindicated in cases of hyperkalemia Hyperkalemia Hyperkalemia is defined as a serum potassium (K+) concentration >5.2 mEq/L. Homeostatic mechanisms maintain the serum K+ concentration between 3.5 and 5.2 mEq/L, despite marked variation in dietary intake. Hyperkalemia can be due to a variety of causes, which include transcellular shifts, tissue breakdown, inadequate renal excretion, and drugs. Hyperkalemia.
Last updated: Jul 10, 2023
Potassium-sparing diuretics Diuretics Agents that promote the excretion of urine through their effects on kidney function. Heart Failure and Angina Medication are medications that act in the principal cells Principal cells Tubular System in the collecting ducts (CDs) to induce diuresis that does not result in excretion of potassium Potassium An element in the alkali group of metals with an atomic symbol k, atomic number 19, and atomic weight 39. 10. It is the chief cation in the intracellular fluid of muscle and other cells. Potassium ion is a strong electrolyte that plays a significant role in the regulation of fluid volume and maintenance of the water-electrolyte balance. Hyperkalemia.
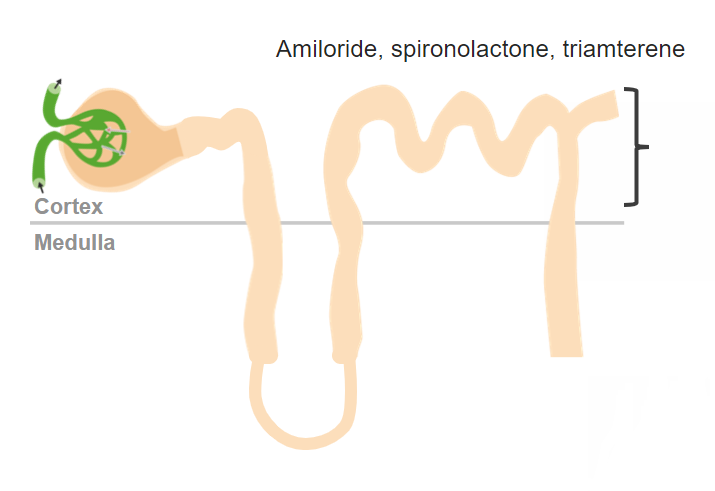
Potassium-sparing diuretics work in the collecting ducts in the nephron.
Image by Lecturio. License: CC BY-NC-SA 4.0| Location of action | Class | Subclasses |
|---|---|---|
| Renal drugs | Drugs affecting the RAAS RAAS A blood pressure regulating system of interacting components that include renin; angiotensinogen; angiotensin converting enzyme; angiotensin i; angiotensin ii; and angiotensinase. Renin, an enzyme produced in the kidney, acts on angiotensinogen, an alpha-2 globulin produced by the liver, forming angiotensin I. Angiotensin-converting enzyme, contained in the lung, acts on angiotensin I in the plasma converting it to angiotensin II, an extremely powerful vasoconstrictor. Angiotensin II causes contraction of the arteriolar and renal vascular smooth muscle, leading to retention of salt and water in the kidney and increased arterial blood pressure. In addition, angiotensin II stimulates the release of aldosterone from the adrenal cortex, which in turn also increases salt and water retention in the kidney. Angiotensin-converting enzyme also breaks down bradykinin, a powerful vasodilator and component of the kallikrein-kinin system. Adrenal Hormones |
|
| Diuretics Diuretics Agents that promote the excretion of urine through their effects on kidney function. Heart Failure and Angina Medication |
|
|
| Extrarenal drugs | Direct vasodilators Vasodilators Drugs used to cause dilation of the blood vessels. Thromboangiitis Obliterans (Buerger Disease) |
|
| Agents acting via the sympathetic nervous system Nervous system The nervous system is a small and complex system that consists of an intricate network of neural cells (or neurons) and even more glial cells (for support and insulation). It is divided according to its anatomical components as well as its functional characteristics. The brain and spinal cord are referred to as the central nervous system, and the branches of nerves from these structures are referred to as the peripheral nervous system. Nervous System: Anatomy, Structure, and Classification |
|
Drugs in this class include:
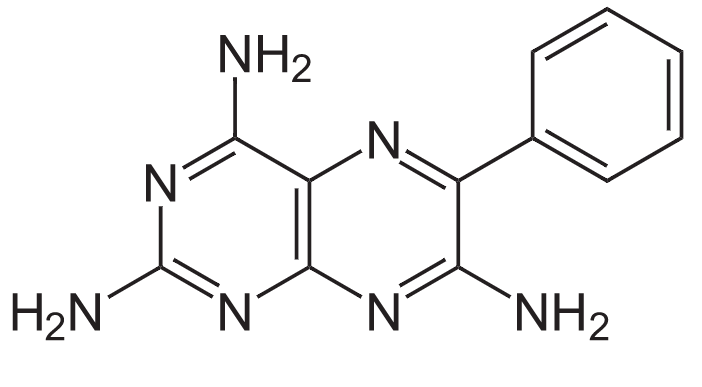
Structure of triamterene
Image: “Structure of Triamterene” by NEUROtiker. License: Public DomainAldosterone antagonists Aldosterone antagonists Drugs that bind to and block the activation of mineralocorticoid receptors by mineralocorticoids such as aldosterone. Heart Failure and Angina Medication (spironolactone, eplerenone):
Sodium channel blockers Sodium Channel Blockers A class of drugs that act by inhibition of sodium influx through cell membranes. Blockade of sodium channels slows the rate and amplitude of initial rapid depolarization, reduces cell excitability, and reduces conduction velocity. Myotonic Dystrophies (triamterene, amiloride Amiloride A pyrazine compound inhibiting sodium reabsorption through sodium channels in renal epithelial cells. This inhibition creates a negative potential in the luminal membranes of principal cells, located in the distal convoluted tubule and collecting duct. Negative potential reduces secretion of potassium and hydrogen ions. Amiloride is used in conjunction with diuretics to spare potassium loss. Liddle Syndrome):
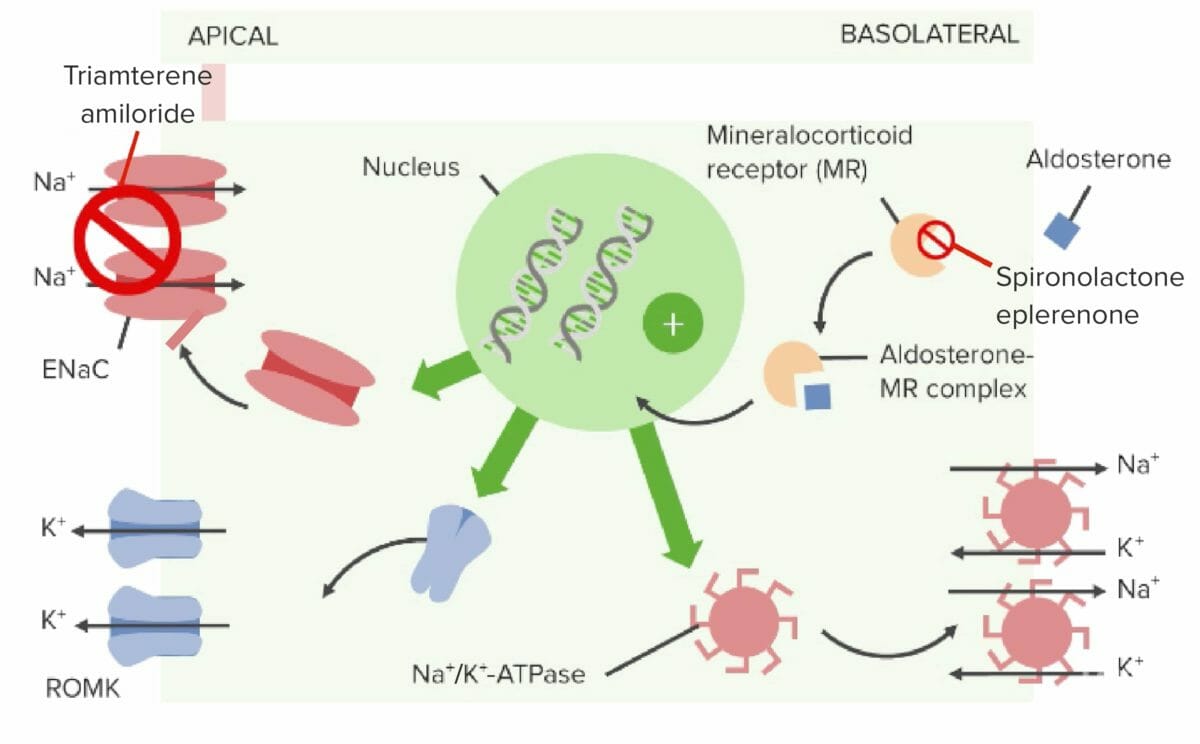
Mechanism of action of aldosterone antagonists and sodium channel blockers
Image by Lecturio.| Drug | Absorption Absorption Absorption involves the uptake of nutrient molecules and their transfer from the lumen of the GI tract across the enterocytes and into the interstitial space, where they can be taken up in the venous or lymphatic circulation. Digestion and Absorption | Distribution | Metabolism | Excretion |
|---|---|---|---|---|
| Spironolactone |
|
Protein binding: > 90% | Rapid and extensive hepatic metabolism to active metabolites |
|
| Eplerenone |
|
|
Hepatic metabolism by CYP3A4 CYP3A4 Class 3 Antiarrhythmic Drugs (Potassium Channel Blockers) to inactive metabolites |
|
| Triamterene |
|
VD: 1.5 L/kg | Hepatic metabolism by CYP1A2 to active metabolites | Excreted renally |
| Amiloride Amiloride A pyrazine compound inhibiting sodium reabsorption through sodium channels in renal epithelial cells. This inhibition creates a negative potential in the luminal membranes of principal cells, located in the distal convoluted tubule and collecting duct. Negative potential reduces secretion of potassium and hydrogen ions. Amiloride is used in conjunction with diuretics to spare potassium loss. Liddle Syndrome |
|
|
Not metabolized |
|
The potassium-sparing diuretics Diuretics Agents that promote the excretion of urine through their effects on kidney function. Heart Failure and Angina Medication are most useful for treating edema Edema Edema is a condition in which excess serous fluid accumulates in the body cavity or interstitial space of connective tissues. Edema is a symptom observed in several medical conditions. It can be categorized into 2 types, namely, peripheral (in the extremities) and internal (in an organ or body cavity). Edema related to states of mineralocorticoid excess Mineralocorticoid excess A hereditary disease characterized by childhood onset hypertension, hypokalemic alkalosis, and low renin and aldosterone secretion. It results from a defect in the activity of the 11-beta-hydroxysteroid dehydrogenase type 2 enzyme which results in inadequate conversion of cortisol to cortisone. The build up of unprocessed cortisol to levels that stimulate mineralocorticoid receptors creates the appearance of having excessive mineralocorticoids. Metabolic Alkalosis, which may be either primary or secondary. Indications include:
Spironolactone has additional indications owing to its antiandrogenic properties:
Some of the other most common diuretics Diuretics Agents that promote the excretion of urine through their effects on kidney function. Heart Failure and Angina Medication include thiazide Thiazide Heterocyclic compounds with sulfur and nitrogen in the ring. This term commonly refers to the benzothiadiazines that inhibit sodium-potassium-chloride symporters and are used as diuretics. Hyponatremia diuretics Diuretics Agents that promote the excretion of urine through their effects on kidney function. Heart Failure and Angina Medication (e.g., hydrochlorothiazide Hydrochlorothiazide A thiazide diuretic often considered the prototypical member of this class. It reduces the reabsorption of electrolytes from the renal tubules. This results in increased excretion of water and electrolytes, including sodium, potassium, chloride, and magnesium. It is used in the treatment of several disorders including edema, hypertension, diabetes insipidus, and hypoparathyroidism. Thiazide Diuretics), loop diuretics Diuretics Agents that promote the excretion of urine through their effects on kidney function. Heart Failure and Angina Medication (e.g., furosemide Furosemide A benzoic-sulfonamide-furan. It is a diuretic with fast onset and short duration that is used for edema and chronic renal insufficiency. Loop Diuretics), carbonic anhydrase Carbonic anhydrase A family of zinc-containing enzymes that catalyze the reversible hydration of carbon dioxide. They play an important role in the transport of carbon dioxide from the tissues to the lung. Carbonic Anhydrase Inhibitors inhibitors (e.g., acetazolamide Acetazolamide One of the carbonic anhydrase inhibitors that is sometimes effective against absence seizures. It is sometimes useful also as an adjunct in the treatment of tonic-clonic, myoclonic, and atonic seizures, particularly in women whose seizures occur or are exacerbated at specific times in the menstrual cycle. However, its usefulness is transient often because of rapid development of tolerance. Its antiepileptic effect may be due to its inhibitory effect on brain carbonic anhydrase, which leads to an increased transneuronal chloride gradient, increased chloride current, and increased inhibition. Carbonic Anhydrase Inhibitors), and osmotic diuretics Diuretics Agents that promote the excretion of urine through their effects on kidney function. Heart Failure and Angina Medication (e.g., mannitol Mannitol A diuretic and renal diagnostic aid related to sorbitol. It has little significant energy value as it is largely eliminated from the body before any metabolism can take place. It can be used to treat oliguria associated with kidney failure or other manifestations of inadequate renal function and has been used for determination of glomerular filtration rate. Mannitol is also commonly used as a research tool in cell biological studies, usually to control osmolarity. Osmotic Diuretics).
| Medication | Mechanism | Physiologic effect | Indication |
|---|---|---|---|
| Thiazide Thiazide Heterocyclic compounds with sulfur and nitrogen in the ring. This term commonly refers to the benzothiadiazines that inhibit sodium-potassium-chloride symporters and are used as diuretics. Hyponatremia diuretic: Hydrochlorothiazide Hydrochlorothiazide A thiazide diuretic often considered the prototypical member of this class. It reduces the reabsorption of electrolytes from the renal tubules. This results in increased excretion of water and electrolytes, including sodium, potassium, chloride, and magnesium. It is used in the treatment of several disorders including edema, hypertension, diabetes insipidus, and hypoparathyroidism. Thiazide Diuretics | ↓ Reabsorption of NaCl in the DCT through the inhibition of Na+/Cl– cotransporter |
|
|
| Loop diuretic: Furosemide Furosemide A benzoic-sulfonamide-furan. It is a diuretic with fast onset and short duration that is used for edema and chronic renal insufficiency. Loop Diuretics | Inhibits the luminal Na+/K+/Cl– cotransporter in the thick ascending limb Thick ascending limb Renal Sodium and Water Regulation of the loop of Henle Loop of Henle The U-shaped portion of the renal tubule in the kidney medulla, consisting of a descending limb and an ascending limb. It is situated between the proximal kidney tubule and the distal kidney tubule. Tubular System |
|
|
| Potassium-sparing diuretic: Spironolactone |
|
|
|
| Carbonic anhydrase inhibitor Carbonic Anhydrase Inhibitor Glaucoma: Acetazolamide Acetazolamide One of the carbonic anhydrase inhibitors that is sometimes effective against absence seizures. It is sometimes useful also as an adjunct in the treatment of tonic-clonic, myoclonic, and atonic seizures, particularly in women whose seizures occur or are exacerbated at specific times in the menstrual cycle. However, its usefulness is transient often because of rapid development of tolerance. Its antiepileptic effect may be due to its inhibitory effect on brain carbonic anhydrase, which leads to an increased transneuronal chloride gradient, increased chloride current, and increased inhibition. Carbonic Anhydrase Inhibitors | Inhibits both the hydration of CO2 in the PCT epithelial cells and the dehydration Dehydration The condition that results from excessive loss of water from a living organism. Volume Depletion and Dehydration of H2CO3 in the PCT lumen; results in ↑ HCO3– and Na+ excretion |
|
|
| Osmotic diuretics Diuretics Agents that promote the excretion of urine through their effects on kidney function. Heart Failure and Angina Medication: Mannitol Mannitol A diuretic and renal diagnostic aid related to sorbitol. It has little significant energy value as it is largely eliminated from the body before any metabolism can take place. It can be used to treat oliguria associated with kidney failure or other manifestations of inadequate renal function and has been used for determination of glomerular filtration rate. Mannitol is also commonly used as a research tool in cell biological studies, usually to control osmolarity. Osmotic Diuretics | ↑ Osmotic pressure Osmotic pressure The pressure required to prevent the passage of solvent through a semipermeable membrane that separates a pure solvent from a solution of the solvent and solute or that separates different concentrations of a solution. It is proportional to the osmolality of the solution. Intravenous Fluids in the glomerular filtrate → ↑ tubular fluid and prevents water reabsorption |
|
|
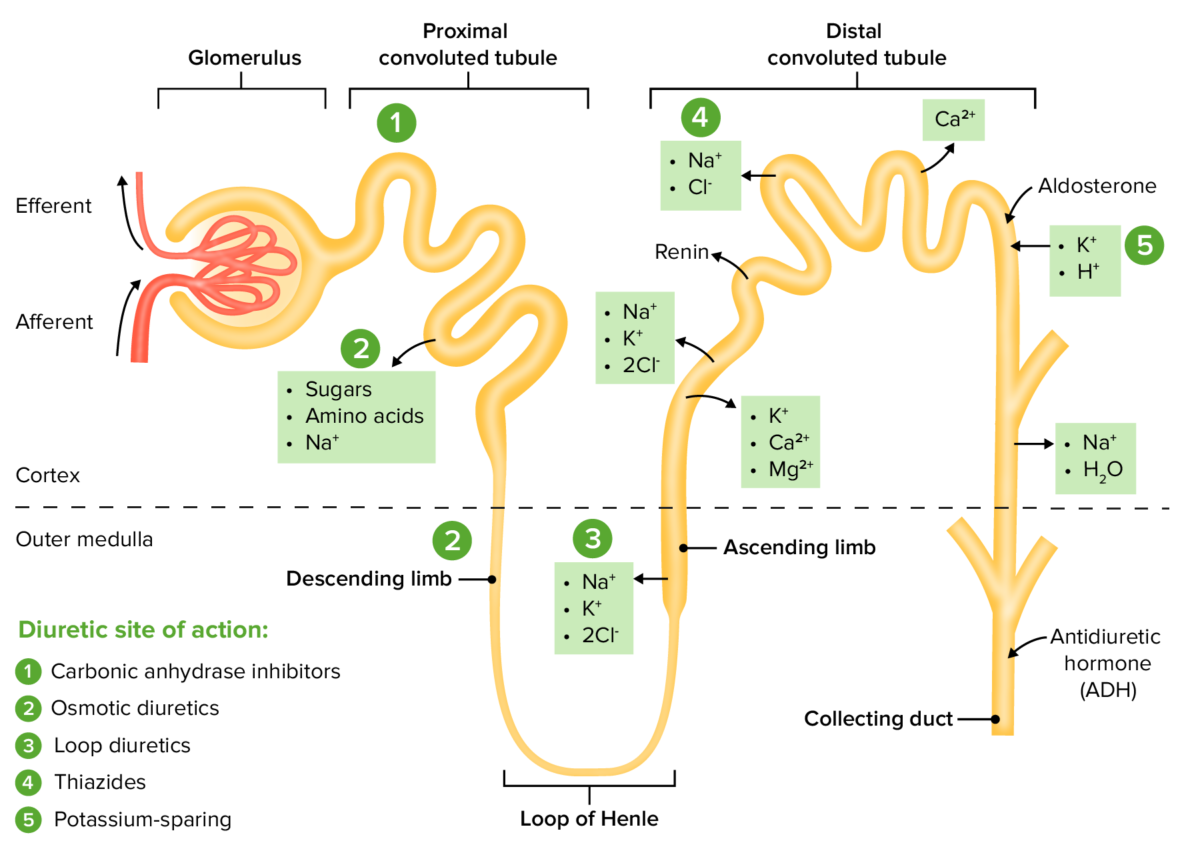
The sites of action within the nephron for the diuretic drug classes
Image by Lecturio. License: CC BY-NC-SA 4.0