Renin-angiotensin-aldosterone system Renin-angiotensin-aldosterone system A blood pressure regulating system of interacting components that include renin; angiotensinogen; angiotensin converting enzyme; angiotensin i; angiotensin ii; and angiotensinase. Renin, an enzyme produced in the kidney, acts on angiotensinogen, an alpha-2 globulin produced by the liver, forming angiotensin I. Angiotensin-converting enzyme, contained in the lung, acts on angiotensin I in the plasma converting it to angiotensin II, an extremely powerful vasoconstrictor. Angiotensin II causes contraction of the arteriolar and renal vascular smooth muscle, leading to retention of salt and water in the kidney and increased arterial blood pressure. In addition, angiotensin II stimulates the release of aldosterone from the adrenal cortex, which in turn also increases salt and water retention in the kidney. Angiotensin-converting enzyme also breaks down bradykinin, a powerful vasodilator and component of the kallikrein-kinin system. Adrenal Hormones inhibitors constitute an important drug class for the treatment of cardiovascular disease. They are 1st-line antihypertensive agents, in addition to being used in the treatment of MI MI MI is ischemia and death of an area of myocardial tissue due to insufficient blood flow and oxygenation, usually from thrombus formation on a ruptured atherosclerotic plaque in the epicardial arteries. Clinical presentation is most commonly with chest pain, but women and patients with diabetes may have atypical symptoms. Myocardial Infarction, heart failure Heart Failure A heterogeneous condition in which the heart is unable to pump out sufficient blood to meet the metabolic need of the body. Heart failure can be caused by structural defects, functional abnormalities (ventricular dysfunction), or a sudden overload beyond its capacity. Chronic heart failure is more common than acute heart failure which results from sudden insult to cardiac function, such as myocardial infarction. Total Anomalous Pulmonary Venous Return (TAPVR), diabetic nephropathy Diabetic nephropathy Kidney injuries associated with diabetes mellitus and affecting kidney glomerulus; arterioles; kidney tubules; and the interstitium. Clinical signs include persistent proteinuria, from microalbuminuria progressing to albuminuria of greater than 300 mg/24 h, leading to reduced glomerular filtration rate and end-stage renal disease. Chronic Diabetic Complications, and stroke. Renin-angiotensin-aldosterone system Renin-angiotensin-aldosterone system A blood pressure regulating system of interacting components that include renin; angiotensinogen; angiotensin converting enzyme; angiotensin i; angiotensin ii; and angiotensinase. Renin, an enzyme produced in the kidney, acts on angiotensinogen, an alpha-2 globulin produced by the liver, forming angiotensin I. Angiotensin-converting enzyme, contained in the lung, acts on angiotensin I in the plasma converting it to angiotensin II, an extremely powerful vasoconstrictor. Angiotensin II causes contraction of the arteriolar and renal vascular smooth muscle, leading to retention of salt and water in the kidney and increased arterial blood pressure. In addition, angiotensin II stimulates the release of aldosterone from the adrenal cortex, which in turn also increases salt and water retention in the kidney. Angiotensin-converting enzyme also breaks down bradykinin, a powerful vasodilator and component of the kallikrein-kinin system. Adrenal Hormones inhibitors include ACEis ACEIs A class of drugs whose main indications are the treatment of hypertension and heart failure. They exert their hemodynamic effect mainly by inhibiting the renin-angiotensin system. They also modulate sympathetic nervous system activity and increase prostaglandin synthesis. They cause mainly vasodilation and mild natriuresis without affecting heart rate and contractility. Heart Failure and Angina Medication, ARBs ARBs Agents that antagonize angiotensin receptors. Many drugs in this class specifically target the angiotensin type 1 receptor. Heart Failure and Angina Medication, direct renin Renin A highly specific (leu-leu) endopeptidase that generates angiotensin I from its precursor angiotensinogen, leading to a cascade of reactions which elevate blood pressure and increase sodium retention by the kidney in the renin-angiotensin system. Renal Sodium and Water Regulation inhibitors (DRIs), angiotensin receptor Receptor Receptors are proteins located either on the surface of or within a cell that can bind to signaling molecules known as ligands (e.g., hormones) and cause some type of response within the cell. Receptors and neprilysin inhibitors (ARNIs), and aldosterone antagonists Aldosterone antagonists Drugs that bind to and block the activation of mineralocorticoid receptors by mineralocorticoids such as aldosterone. Heart Failure and Angina Medication, which affect different components of the RAAS RAAS A blood pressure regulating system of interacting components that include renin; angiotensinogen; angiotensin converting enzyme; angiotensin i; angiotensin ii; and angiotensinase. Renin, an enzyme produced in the kidney, acts on angiotensinogen, an alpha-2 globulin produced by the liver, forming angiotensin I. Angiotensin-converting enzyme, contained in the lung, acts on angiotensin I in the plasma converting it to angiotensin II, an extremely powerful vasoconstrictor. Angiotensin II causes contraction of the arteriolar and renal vascular smooth muscle, leading to retention of salt and water in the kidney and increased arterial blood pressure. In addition, angiotensin II stimulates the release of aldosterone from the adrenal cortex, which in turn also increases salt and water retention in the kidney. Angiotensin-converting enzyme also breaks down bradykinin, a powerful vasodilator and component of the kallikrein-kinin system. Adrenal Hormones pathway. In general, the use of RAAS RAAS A blood pressure regulating system of interacting components that include renin; angiotensinogen; angiotensin converting enzyme; angiotensin i; angiotensin ii; and angiotensinase. Renin, an enzyme produced in the kidney, acts on angiotensinogen, an alpha-2 globulin produced by the liver, forming angiotensin I. Angiotensin-converting enzyme, contained in the lung, acts on angiotensin I in the plasma converting it to angiotensin II, an extremely powerful vasoconstrictor. Angiotensin II causes contraction of the arteriolar and renal vascular smooth muscle, leading to retention of salt and water in the kidney and increased arterial blood pressure. In addition, angiotensin II stimulates the release of aldosterone from the adrenal cortex, which in turn also increases salt and water retention in the kidney. Angiotensin-converting enzyme also breaks down bradykinin, a powerful vasodilator and component of the kallikrein-kinin system. Adrenal Hormones inhibitors results in decreased vasoconstriction Vasoconstriction The physiological narrowing of blood vessels by contraction of the vascular smooth muscle. Vascular Resistance, Flow, and Mean Arterial Pressure and serum blood volume. Common adverse effects include hyperkalemia Hyperkalemia Hyperkalemia is defined as a serum potassium (K+) concentration >5.2 mEq/L. Homeostatic mechanisms maintain the serum K+ concentration between 3.5 and 5.2 mEq/L, despite marked variation in dietary intake. Hyperkalemia can be due to a variety of causes, which include transcellular shifts, tissue breakdown, inadequate renal excretion, and drugs. Hyperkalemia, cough, angioedema Angioedema Angioedema is a localized, self-limited (but potentially life-threatening), nonpitting, asymmetrical edema occurring in the deep layers of the skin and mucosal tissue. The common underlying pathophysiology involves inflammatory mediators triggering significant vasodilation and increased capillary permeability. Angioedema, and pancreatitis Pancreatitis Inflammation of the pancreas. Pancreatitis is classified as acute unless there are computed tomographic or endoscopic retrograde cholangiopancreatographic findings of chronic pancreatitis. The two most common forms of acute pancreatitis are alcoholic pancreatitis and gallstone pancreatitis. Acute Pancreatitis, which are all more common with the use of ACEis ACEIs A class of drugs whose main indications are the treatment of hypertension and heart failure. They exert their hemodynamic effect mainly by inhibiting the renin-angiotensin system. They also modulate sympathetic nervous system activity and increase prostaglandin synthesis. They cause mainly vasodilation and mild natriuresis without affecting heart rate and contractility. Heart Failure and Angina Medication than ARBs ARBs Agents that antagonize angiotensin receptors. Many drugs in this class specifically target the angiotensin type 1 receptor. Heart Failure and Angina Medication.
Last updated: Jul 10, 2023
Renin-angiotensin-aldosterone system Renin-angiotensin-aldosterone system A blood pressure regulating system of interacting components that include renin; angiotensinogen; angiotensin converting enzyme; angiotensin i; angiotensin ii; and angiotensinase. Renin, an enzyme produced in the kidney, acts on angiotensinogen, an alpha-2 globulin produced by the liver, forming angiotensin I. Angiotensin-converting enzyme, contained in the lung, acts on angiotensin I in the plasma converting it to angiotensin II, an extremely powerful vasoconstrictor. Angiotensin II causes contraction of the arteriolar and renal vascular smooth muscle, leading to retention of salt and water in the kidney and increased arterial blood pressure. In addition, angiotensin II stimulates the release of aldosterone from the adrenal cortex, which in turn also increases salt and water retention in the kidney. Angiotensin-converting enzyme also breaks down bradykinin, a powerful vasodilator and component of the kallikrein-kinin system. Adrenal Hormones inhibitors constitute an important class of medications for the treatment of cardiovascular diseases, and are 1st-line agents for the treatment of hypertension Hypertension Hypertension, or high blood pressure, is a common disease that manifests as elevated systemic arterial pressures. Hypertension is most often asymptomatic and is found incidentally as part of a routine physical examination or during triage for an unrelated medical encounter. Hypertension.
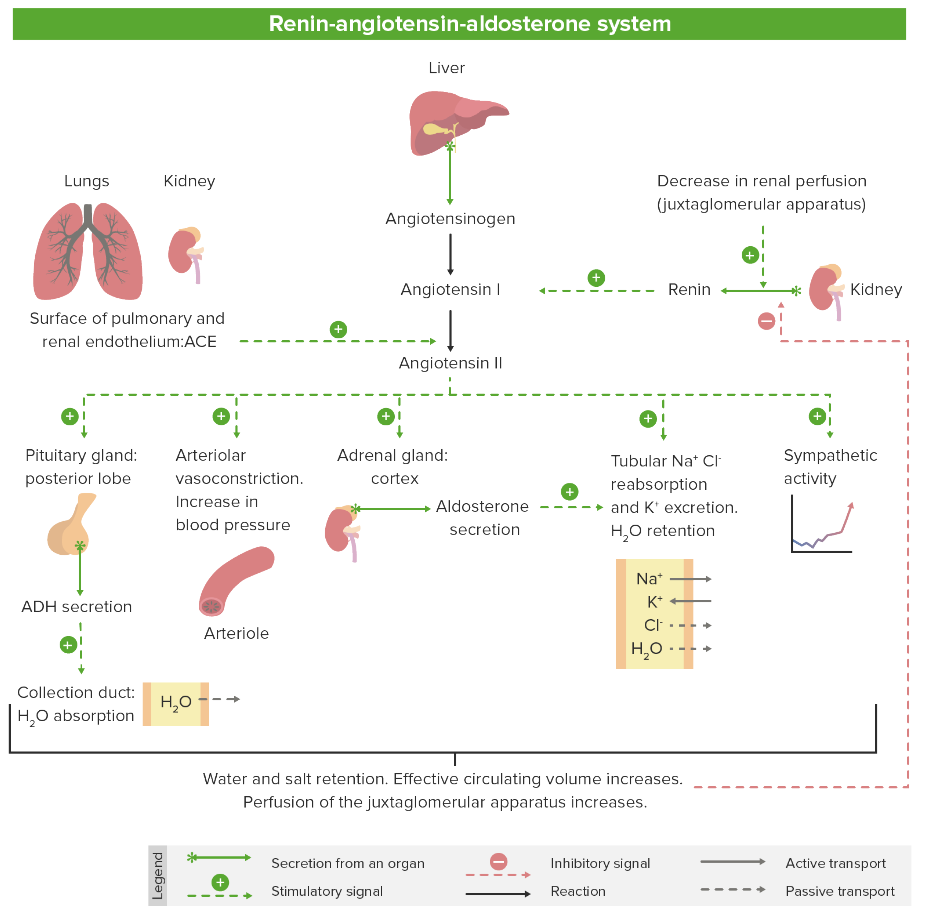
Overview of the RAAS
Image by Lecturio. License: CC BY-NC-SA 4.0Drugs in the RAAS RAAS A blood pressure regulating system of interacting components that include renin; angiotensinogen; angiotensin converting enzyme; angiotensin i; angiotensin ii; and angiotensinase. Renin, an enzyme produced in the kidney, acts on angiotensinogen, an alpha-2 globulin produced by the liver, forming angiotensin I. Angiotensin-converting enzyme, contained in the lung, acts on angiotensin I in the plasma converting it to angiotensin II, an extremely powerful vasoconstrictor. Angiotensin II causes contraction of the arteriolar and renal vascular smooth muscle, leading to retention of salt and water in the kidney and increased arterial blood pressure. In addition, angiotensin II stimulates the release of aldosterone from the adrenal cortex, which in turn also increases salt and water retention in the kidney. Angiotensin-converting enzyme also breaks down bradykinin, a powerful vasodilator and component of the kallikrein-kinin system. Adrenal Hormones inhibitor class include:
| Location of action | Class | Subclass |
|---|---|---|
| Renal | Drugs affecting the RAAS RAAS A blood pressure regulating system of interacting components that include renin; angiotensinogen; angiotensin converting enzyme; angiotensin i; angiotensin ii; and angiotensinase. Renin, an enzyme produced in the kidney, acts on angiotensinogen, an alpha-2 globulin produced by the liver, forming angiotensin I. Angiotensin-converting enzyme, contained in the lung, acts on angiotensin I in the plasma converting it to angiotensin II, an extremely powerful vasoconstrictor. Angiotensin II causes contraction of the arteriolar and renal vascular smooth muscle, leading to retention of salt and water in the kidney and increased arterial blood pressure. In addition, angiotensin II stimulates the release of aldosterone from the adrenal cortex, which in turn also increases salt and water retention in the kidney. Angiotensin-converting enzyme also breaks down bradykinin, a powerful vasodilator and component of the kallikrein-kinin system. Adrenal Hormones |
|
| Diuretics Diuretics Agents that promote the excretion of urine through their effects on kidney function. Heart Failure and Angina Medication |
|
|
| Extrarenal | Direct vasodilators Vasodilators Drugs used to cause dilation of the blood vessels. Thromboangiitis Obliterans (Buerger Disease) |
|
| Agents acting via the sympathetic nervous system Nervous system The nervous system is a small and complex system that consists of an intricate network of neural cells (or neurons) and even more glial cells (for support and insulation). It is divided according to its anatomical components as well as its functional characteristics. The brain and spinal cord are referred to as the central nervous system, and the branches of nerves from these structures are referred to as the peripheral nervous system. Nervous System: Anatomy, Structure, and Classification |
|
All drugs within a class have the same basic structure but different functional groups attached, which explains their different pharmacokinetic and safety profiles.
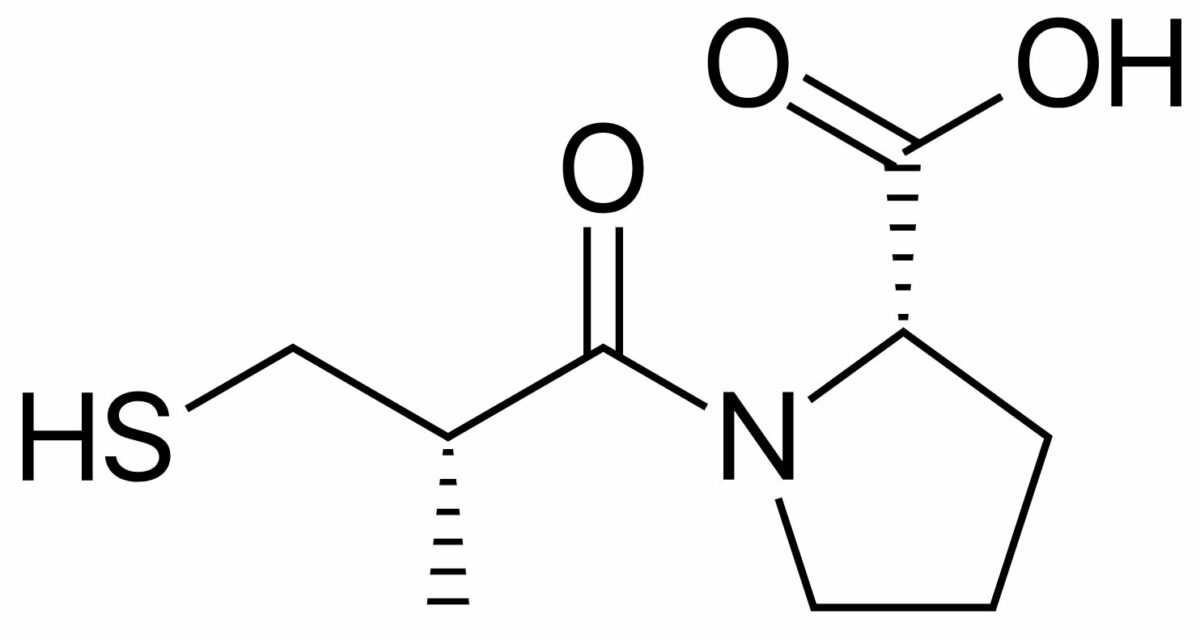
Chemical structure of captopril (brand name Capoten), a widely used ACEi
Image: “Captopril structure” by Vaccinationist. License: Public Domain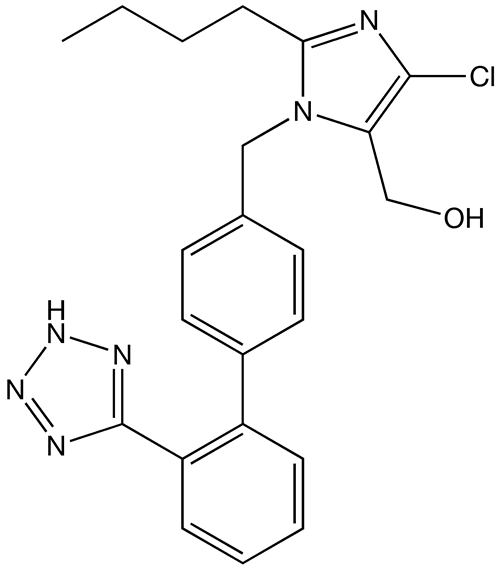
Chemical structure of losartan, a widely used ARB
Image: “Losartan structure” by Techelf. License: Public Domain| Drug class | Mechanism of Action | Physiologic effects |
|---|---|---|
| ACEis ACEIs A class of drugs whose main indications are the treatment of hypertension and heart failure. They exert their hemodynamic effect mainly by inhibiting the renin-angiotensin system. They also modulate sympathetic nervous system activity and increase prostaglandin synthesis. They cause mainly vasodilation and mild natriuresis without affecting heart rate and contractility. Heart Failure and Angina Medication | Inhibit ACE, preventing:
|
Decreased peripheral vascular
resistance
Resistance
Physiologically, the opposition to flow of air caused by the forces of friction. As a part of pulmonary function testing, it is the ratio of driving pressure to the rate of air flow.
Ventilation: Mechanics of Breathing via:
|
| ARBs ARBs Agents that antagonize angiotensin receptors. Many drugs in this class specifically target the angiotensin type 1 receptor. Heart Failure and Angina Medication | Inhibit angiotensin-1 receptors Receptors Receptors are proteins located either on the surface of or within a cell that can bind to signaling molecules known as ligands (e.g., hormones) and cause some type of response within the cell. Receptors, preventing them from exerting their effects |
|
| DRis | Directly inhibits renin Renin A highly specific (leu-leu) endopeptidase that generates angiotensin I from its precursor angiotensinogen, leading to a cascade of reactions which elevate blood pressure and increase sodium retention by the kidney in the renin-angiotensin system. Renal Sodium and Water Regulation activity, blocking the conversion of angiotensinogen to angiotensin I | ↓ Angiotensin I, angiotensin II Angiotensin II An octapeptide that is a potent but labile vasoconstrictor. It is produced from angiotensin I after the removal of two amino acids at the c-terminal by angiotensin converting enzyme. The amino acid in position 5 varies in different species. To block vasoconstriction and hypertension effect of angiotensin II, patients are often treated with ace inhibitors or with angiotensin II type 1 receptor blockers. Renal Sodium and Water Regulation, and aldosterone Aldosterone A hormone secreted by the adrenal cortex that regulates electrolyte and water balance by increasing the renal retention of sodium and the excretion of potassium. Hyperkalemia → ↓ peripheral vascular resistance Resistance Physiologically, the opposition to flow of air caused by the forces of friction. As a part of pulmonary function testing, it is the ratio of driving pressure to the rate of air flow. Ventilation: Mechanics of Breathing |
| ARNIs | Combination of ARB and a neprilysin inhibitor:
|
|
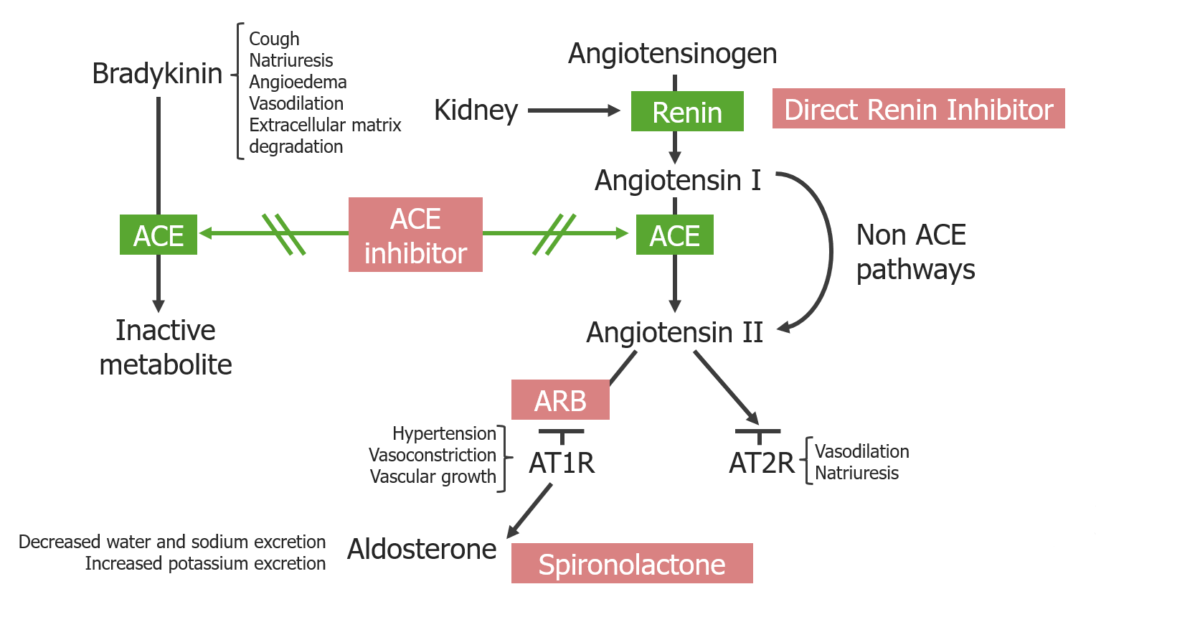
Overview of RAAS inhibitors and their location of action:
ACE inhibitors block both the degradation of bradykinin and the generation of angiotensin II.
ARBs block the angiotensin II type 1 receptors. Direct renin inhibitors block the generation of angiotensin I.
Spironolactone blocks mineralocorticoid receptors at the principal cells in the distal renal tubules and cortical collecting duct.
| Drug | Absorption Absorption Absorption involves the uptake of nutrient molecules and their transfer from the lumen of the GI tract across the enterocytes and into the interstitial space, where they can be taken up in the venous or lymphatic circulation. Digestion and Absorption | Distribution | Metabolism | Excretion |
|---|---|---|---|---|
| ACEis ACEIs A class of drugs whose main indications are the treatment of hypertension and heart failure. They exert their hemodynamic effect mainly by inhibiting the renin-angiotensin system. They also modulate sympathetic nervous system activity and increase prostaglandin synthesis. They cause mainly vasodilation and mild natriuresis without affecting heart rate and contractility. Heart Failure and Angina Medication |
|
Most drugs have minimal protein binding and relatively modest volumes of distribution | Prodrugs are activated via hydrolysis Hydrolysis The process of cleaving a chemical compound by the addition of a molecule of water. Proteins and Peptides in the liver Liver The liver is the largest gland in the human body. The liver is found in the superior right quadrant of the abdomen and weighs approximately 1.5 kilograms. Its main functions are detoxification, metabolism, nutrient storage (e.g., iron and vitamins), synthesis of coagulation factors, formation of bile, filtration, and storage of blood. Liver: Anatomy; active drugs are unchanged. |
|
| ARBs ARBs Agents that antagonize angiotensin receptors. Many drugs in this class specifically target the angiotensin type 1 receptor. Heart Failure and Angina Medication |
|
|
Hepatic metabolism |
|
| DRI: aliskiren |
|
Protein binding: 50% | Extent of hepatic metabolism unknown |
|
The most common adverse effects of ACEis ACEIs A class of drugs whose main indications are the treatment of hypertension and heart failure. They exert their hemodynamic effect mainly by inhibiting the renin-angiotensin system. They also modulate sympathetic nervous system activity and increase prostaglandin synthesis. They cause mainly vasodilation and mild natriuresis without affecting heart rate and contractility. Heart Failure and Angina Medication and ARBs ARBs Agents that antagonize angiotensin receptors. Many drugs in this class specifically target the angiotensin type 1 receptor. Heart Failure and Angina Medication are shown in the table.
| Symptom | ACEis ACEIs A class of drugs whose main indications are the treatment of hypertension and heart failure. They exert their hemodynamic effect mainly by inhibiting the renin-angiotensin system. They also modulate sympathetic nervous system activity and increase prostaglandin synthesis. They cause mainly vasodilation and mild natriuresis without affecting heart rate and contractility. Heart Failure and Angina Medication | ARBs ARBs Agents that antagonize angiotensin receptors. Many drugs in this class specifically target the angiotensin type 1 receptor. Heart Failure and Angina Medication (and ARNIs) |
|---|---|---|
| Hyperkalemia Hyperkalemia Hyperkalemia is defined as a serum potassium (K+) concentration >5.2 mEq/L. Homeostatic mechanisms maintain the serum K+ concentration between 3.5 and 5.2 mEq/L, despite marked variation in dietary intake. Hyperkalemia can be due to a variety of causes, which include transcellular shifts, tissue breakdown, inadequate renal excretion, and drugs. Hyperkalemia | 1% | 0.3% |
| Cough | 10%‒20% | 1 per 1,000 |
| Pancreatitis Pancreatitis Inflammation of the pancreas. Pancreatitis is classified as acute unless there are computed tomographic or endoscopic retrograde cholangiopancreatographic findings of chronic pancreatitis. The two most common forms of acute pancreatitis are alcoholic pancreatitis and gallstone pancreatitis. Acute Pancreatitis | 1 per 5,000 |
|
| Angioedema Angioedema Angioedema is a localized, self-limited (but potentially life-threatening), nonpitting, asymmetrical edema occurring in the deep layers of the skin and mucosal tissue. The common underlying pathophysiology involves inflammatory mediators triggering significant vasodilation and increased capillary permeability. Angioedema | 1 per 2,000 | 1 per 20,000 |
Additional side effects may include:
| Medications | Mechanisms | Physiologic effects | Indications |
|---|---|---|---|
| ACEis ACEIs A class of drugs whose main indications are the treatment of hypertension and heart failure. They exert their hemodynamic effect mainly by inhibiting the renin-angiotensin system. They also modulate sympathetic nervous system activity and increase prostaglandin synthesis. They cause mainly vasodilation and mild natriuresis without affecting heart rate and contractility. Heart Failure and Angina Medication | Inhibit ACE, which:
|
|
|
| ARBs ARBs Agents that antagonize angiotensin receptors. Many drugs in this class specifically target the angiotensin type 1 receptor. Heart Failure and Angina Medication | Inhibit angiotensin-1 receptors Receptors Receptors are proteins located either on the surface of or within a cell that can bind to signaling molecules known as ligands (e.g., hormones) and cause some type of response within the cell. Receptors, preventing them from exerting their effects |
|
|
| Calcium Calcium A basic element found in nearly all tissues. It is a member of the alkaline earth family of metals with the atomic symbol ca, atomic number 20, and atomic weight 40. Calcium is the most abundant mineral in the body and combines with phosphorus to form calcium phosphate in the bones and teeth. It is essential for the normal functioning of nerves and muscles and plays a role in blood coagulation (as factor IV) and in many enzymatic processes. Electrolytes channel blockers | Bind BIND Hyperbilirubinemia of the Newborn to and inhibit L-type calcium Calcium A basic element found in nearly all tissues. It is a member of the alkaline earth family of metals with the atomic symbol ca, atomic number 20, and atomic weight 40. Calcium is the most abundant mineral in the body and combines with phosphorus to form calcium phosphate in the bones and teeth. It is essential for the normal functioning of nerves and muscles and plays a role in blood coagulation (as factor IV) and in many enzymatic processes. Electrolytes channels Channels The Cell: Cell Membrane in cardiac myocytes Myocytes Mature contractile cells, commonly known as myocytes, that form one of three kinds of muscle. The three types of muscle cells are skeletal, cardiac, and smooth. They are derived from embryonic (precursor) muscle cells called myoblasts. Muscle Tissue: Histology and vascular smooth muscle |
|
|
| Beta blockers | Inhibit β catecholamine receptors Receptors Receptors are proteins located either on the surface of or within a cell that can bind to signaling molecules known as ligands (e.g., hormones) and cause some type of response within the cell. Receptors with varying affinity for the β1 vs β2 receptors Receptors Receptors are proteins located either on the surface of or within a cell that can bind to signaling molecules known as ligands (e.g., hormones) and cause some type of response within the cell. Receptors |
|
|
| Thiazide Thiazide Heterocyclic compounds with sulfur and nitrogen in the ring. This term commonly refers to the benzothiadiazines that inhibit sodium-potassium-chloride symporters and are used as diuretics. Hyponatremia diuretics Diuretics Agents that promote the excretion of urine through their effects on kidney function. Heart Failure and Angina Medication | ↓ Reabsorption of NaCl in the distal convoluted tubule Distal convoluted tubule The portion of renal tubule that begins from the enlarged segment of the ascending limb of the loop of henle. It reenters the kidney cortex and forms the convoluted segments of the distal tubule. Gitelman Syndrome through inhibition of the Na+/ chloride Chloride Inorganic compounds derived from hydrochloric acid that contain the Cl- ion. Electrolytes cotransporter |
|
|
| Loop diuretics Diuretics Agents that promote the excretion of urine through their effects on kidney function. Heart Failure and Angina Medication | Inhibit the luminal Na+/K+/ chloride Chloride Inorganic compounds derived from hydrochloric acid that contain the Cl- ion. Electrolytes cotransporter in the thick ascending limb Thick ascending limb Renal Sodium and Water Regulation of the loop of Henle Loop of Henle The U-shaped portion of the renal tubule in the kidney medulla, consisting of a descending limb and an ascending limb. It is situated between the proximal kidney tubule and the distal kidney tubule. Tubular System |
|
|
| K+-sparing diuretics Diuretics Agents that promote the excretion of urine through their effects on kidney function. Heart Failure and Angina Medication |
|
|
|