The adrenal glands are a pair of retroperitoneal Retroperitoneal Peritoneum: Anatomy endocrine glands Endocrine glands Ductless glands that secrete hormones directly into the blood circulation. These hormones influence the metabolism and other functions of cells in the body. Glandular Epithelium: Histology located above the kidneys Kidneys The kidneys are a pair of bean-shaped organs located retroperitoneally against the posterior wall of the abdomen on either side of the spine. As part of the urinary tract, the kidneys are responsible for blood filtration and excretion of water-soluble waste in the urine. Kidneys: Anatomy. The outer parenchyma is called the adrenal cortex and has 3 distinct zones, each with its own secretory products. The outer zona glomerulosa secretes mineralocorticoids Mineralocorticoids Mineralocorticoids are a drug class within the corticosteroid family and fludrocortisone is the primary medication within this class. Fludrocortisone is a fluorinated analog of cortisone. The fluorine moiety protects the drug from isoenzyme inactivation in the kidney, allowing it to exert its mineralocorticoid effect. Mineralocorticoids (primarily aldosterone Aldosterone A hormone secreted by the adrenal cortex that regulates electrolyte and water balance by increasing the renal retention of sodium and the excretion of potassium. Hyperkalemia); the middle zona fasciculata secretes glucocorticoids Glucocorticoids Glucocorticoids are a class within the corticosteroid family. Glucocorticoids are chemically and functionally similar to endogenous cortisol. There are a wide array of indications, which primarily benefit from the antiinflammatory and immunosuppressive effects of this class of drugs. Glucocorticoids (primarily cortisol Cortisol Glucocorticoids); and the innermost zona reticularis secretes androgens Androgens Androgens are naturally occurring steroid hormones responsible for development and maintenance of the male sex characteristics, including penile, scrotal, and clitoral growth, development of sexual hair, deepening of the voice, and musculoskeletal growth. Androgens and Antiandrogens. Beneath the cortex lies the adrenal medulla, which secretes catecholamines Catecholamines A general class of ortho-dihydroxyphenylalkylamines derived from tyrosine. Adrenal Hormones involved in the fight-or-flight response. The medullary cells function more like neurosecretory postsynaptic neurons Postsynaptic neurons Autonomic Nervous System: Anatomy rather than traditional endocrine cells. The adrenal glands have a rich vascular supply and complex relationships with their surrounding organs.
Last updated: Jan 9, 2025
There are 2 adrenal glands in the body and each gland is in close association with a kidney. The adrenal glands are:
Adrenal gland located on the superior medial aspect of each kidney
Image by Lecturio.The adrenal glands are composed of 2 functionally separate layers: the adrenal cortex and the adrenal medulla. Each layer has its own embryologic origins, anatomy, histology, and functions.
The adrenal cortex has 3 distinct zones, and each zone has its own regulation and secretory products. These 3 zones (from external to internal) are:
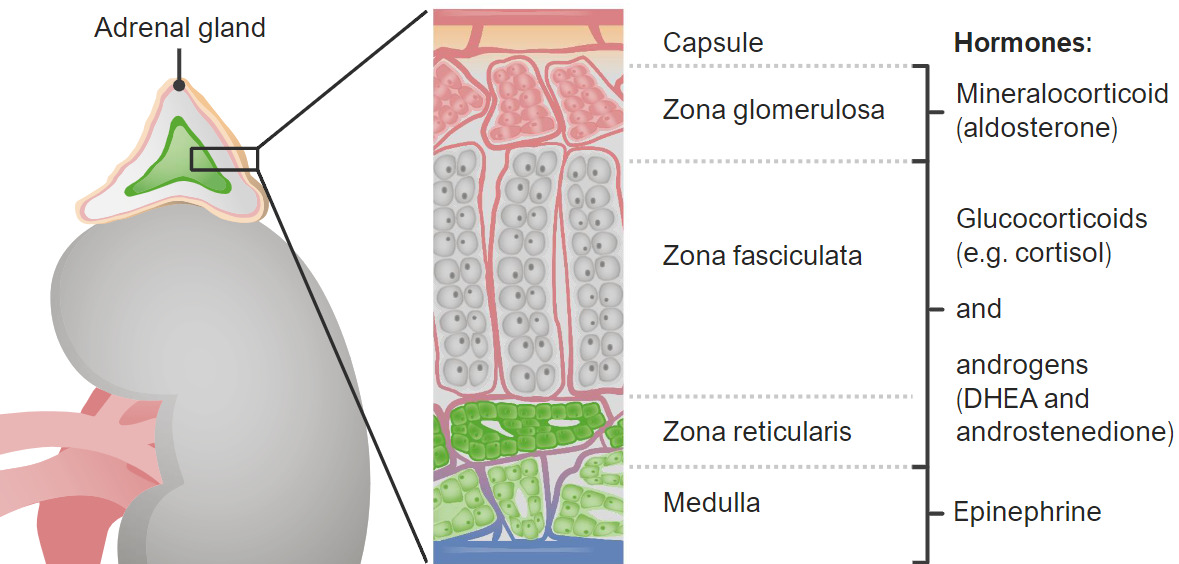
Zones of the adrenal cortex and medulla and their hormonal products
DHEA: dehydroepiandrosterone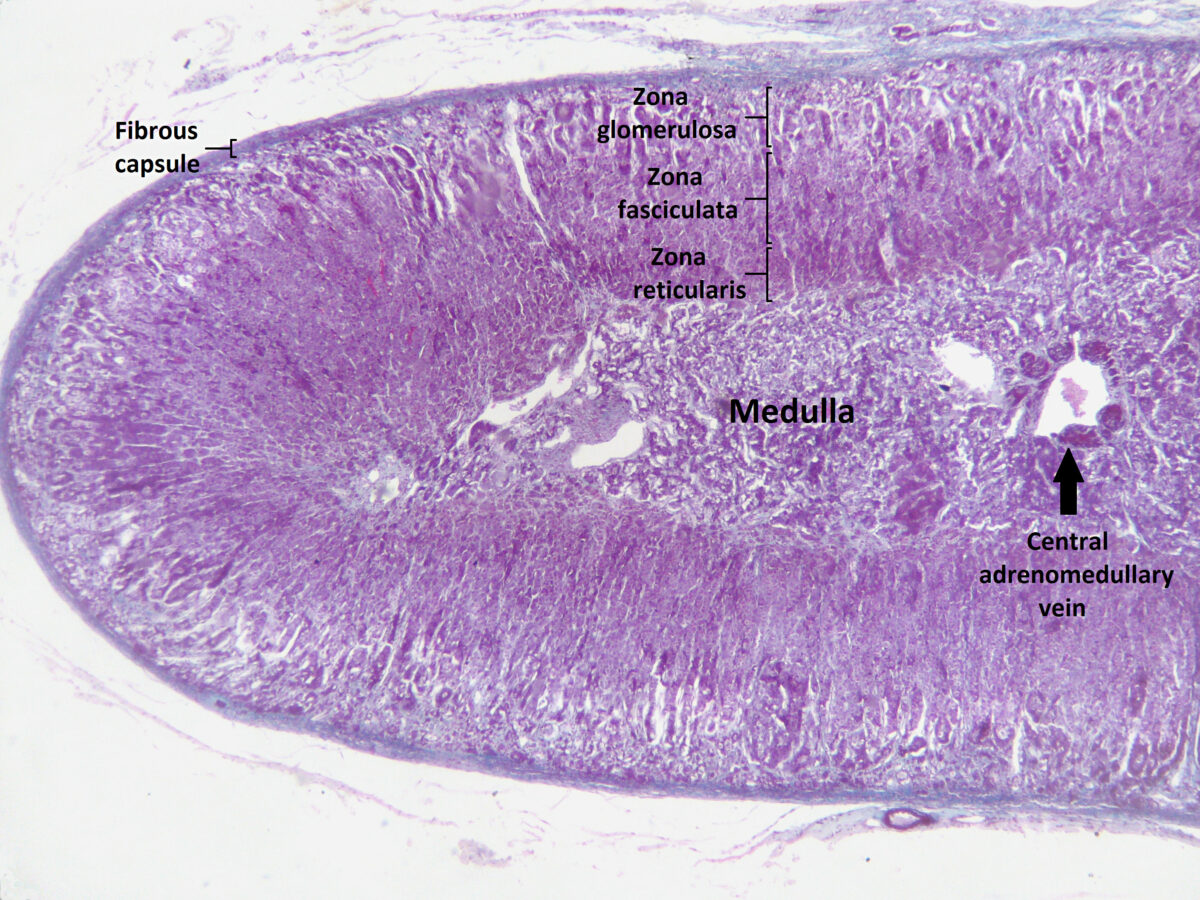
Section of the human adrenal gland under the microscope showing its different layers from the surface to the center: zona glomerulosa, zona fasciculata, zona reticularis, medulla. In the medulla, the central adrenomedullary vein is visible.
Image: “Section of human adrenal gland under the microscope” by Jpogi. License: CC0 1.0Adrenal glands are highly vascular organs.
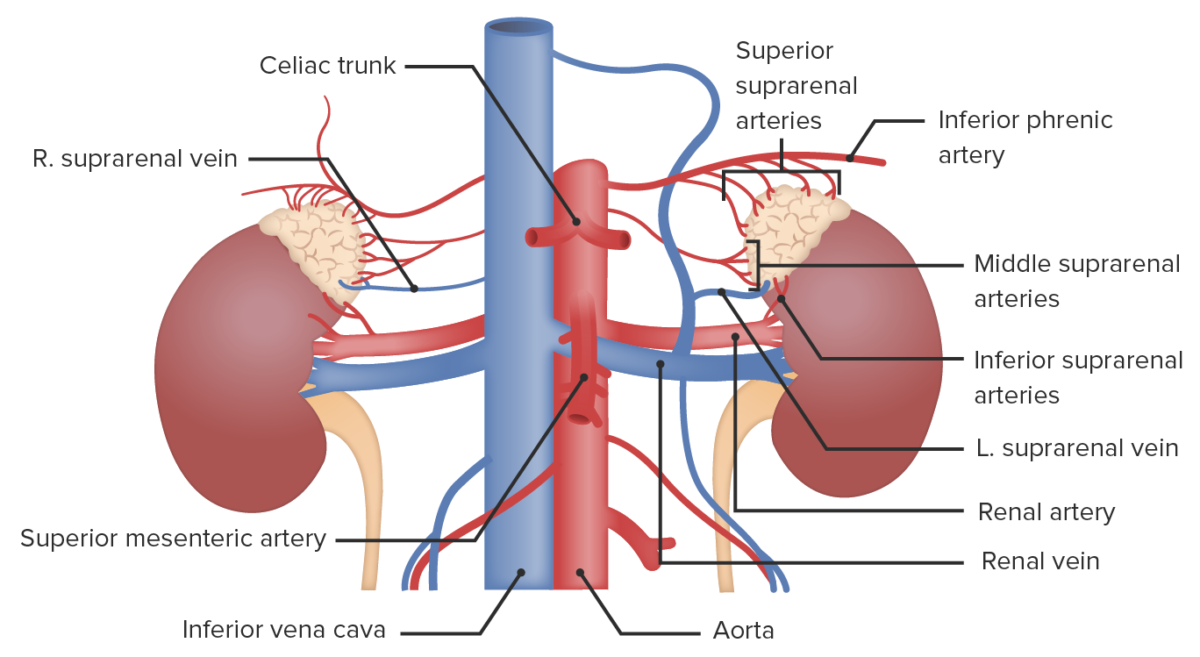
Vascular supply to the adrenal glands
Image by Lecturio.The adrenal cortex secretes hormones Hormones Hormones are messenger molecules that are synthesized in one part of the body and move through the bloodstream to exert specific regulatory effects on another part of the body. Hormones play critical roles in coordinating cellular activities throughout the body in response to the constant changes in both the internal and external environments. Hormones: Overview and Types that are involved in the regulation of fluids, electrolytes Electrolytes Electrolytes are mineral salts that dissolve in water and dissociate into charged particles called ions, which can be either be positively (cations) or negatively (anions) charged. Electrolytes are distributed in the extracellular and intracellular compartments in different concentrations. Electrolytes are essential for various basic life-sustaining functions. Electrolytes, available energy, and inflammation Inflammation Inflammation is a complex set of responses to infection and injury involving leukocytes as the principal cellular mediators in the body’s defense against pathogenic organisms. Inflammation is also seen as a response to tissue injury in the process of wound healing. The 5 cardinal signs of inflammation are pain, heat, redness, swelling, and loss of function. Inflammation, and in the development of sexual characteristics.
The adrenal medulla secretes the catecholamines Catecholamines A general class of ortho-dihydroxyphenylalkylamines derived from tyrosine. Adrenal Hormones epinephrine Epinephrine The active sympathomimetic hormone from the adrenal medulla. It stimulates both the alpha- and beta- adrenergic systems, causes systemic vasoconstriction and gastrointestinal relaxation, stimulates the heart, and dilates bronchi and cerebral vessels. Sympathomimetic Drugs and norepinephrine Norepinephrine Precursor of epinephrine that is secreted by the adrenal medulla and is a widespread central and autonomic neurotransmitter. Norepinephrine is the principal transmitter of most postganglionic sympathetic fibers, and of the diffuse projection system in the brain that arises from the locus ceruleus. Receptors and Neurotransmitters of the CNS, which stimulate the fight-or-flight response. The effects include:
Several clinical conditions can result from abnormalities of the adrenal hormones Adrenal hormones There are 2 primary portions of the adrenal glands, the adrenal medulla and the adrenal cortex. The adrenal medulla is the inner portion of the gland, secreting epinephrine and, to a lesser degree, norepinephrine. The adrenal cortex is the outer portion of the gland and secretes mineralocorticoids, glucocorticoids, and androgens. Adrenal Hormones. The most important conditions include: