Glycogen is a branched polymer and the storage form of carbohydrates Carbohydrates A class of organic compounds composed of carbon, hydrogen, and oxygen in a ratio of cn(H2O)n. The largest class of organic compounds, including starch; glycogen; cellulose; polysaccharides; and simple monosaccharides. Basics of Carbohydrates in the human body. Major sites of storage are the liver Liver The liver is the largest gland in the human body. The liver is found in the superior right quadrant of the abdomen and weighs approximately 1.5 kilograms. Its main functions are detoxification, metabolism, nutrient storage (e.g., iron and vitamins), synthesis of coagulation factors, formation of bile, filtration, and storage of blood. Liver: Anatomy and skeletal muscles Skeletal muscles A subtype of striated muscle, attached by tendons to the skeleton. Skeletal muscles are innervated and their movement can be consciously controlled. They are also called voluntary muscles. Muscle Tissue: Histology. Glycogen is the main source of energy during fasting or in between meals. Glycogen provides energy for up to 18 hours, after which energy requirements are met MET Preoperative Care by fatty acid oxidation. The 2 metabolic pathways of glycogen are glycogenesis (glycogen synthesis Synthesis Polymerase Chain Reaction (PCR)) and glycogenolysis (glycogen breakdown). The key regulatory enzymes Enzymes Enzymes are complex protein biocatalysts that accelerate chemical reactions without being consumed by them. Due to the body's constant metabolic needs, the absence of enzymes would make life unsustainable, as reactions would occur too slowly without these molecules. Basics of Enzymes in these processes are glycogen synthase (in glycogenesis) and glycogen phosphorylase (in glycogenolysis). These pathways proceed depending on the energy needs of the cells, generally modulated by hormonal and allosteric regulators. Abnormal accumulation of glycogen occurs with enzyme deficiencies causing different types of glycogen storage disorders Glycogen storage disorders Glycogen storage disorders (GSDs) are genetic defects leading to disorders of carbohydrate metabolism. The disorders are caused by pathogenic variants in genes that affect enzymes involved in glycogen breakdown. Deficiency of 1 of these enzymes may occur in the liver or muscles and can cause hypoglycemia and/or abnormal glycogen deposition in tissues. Glycogen Storage Disorders.
Last updated: Apr 25, 2025
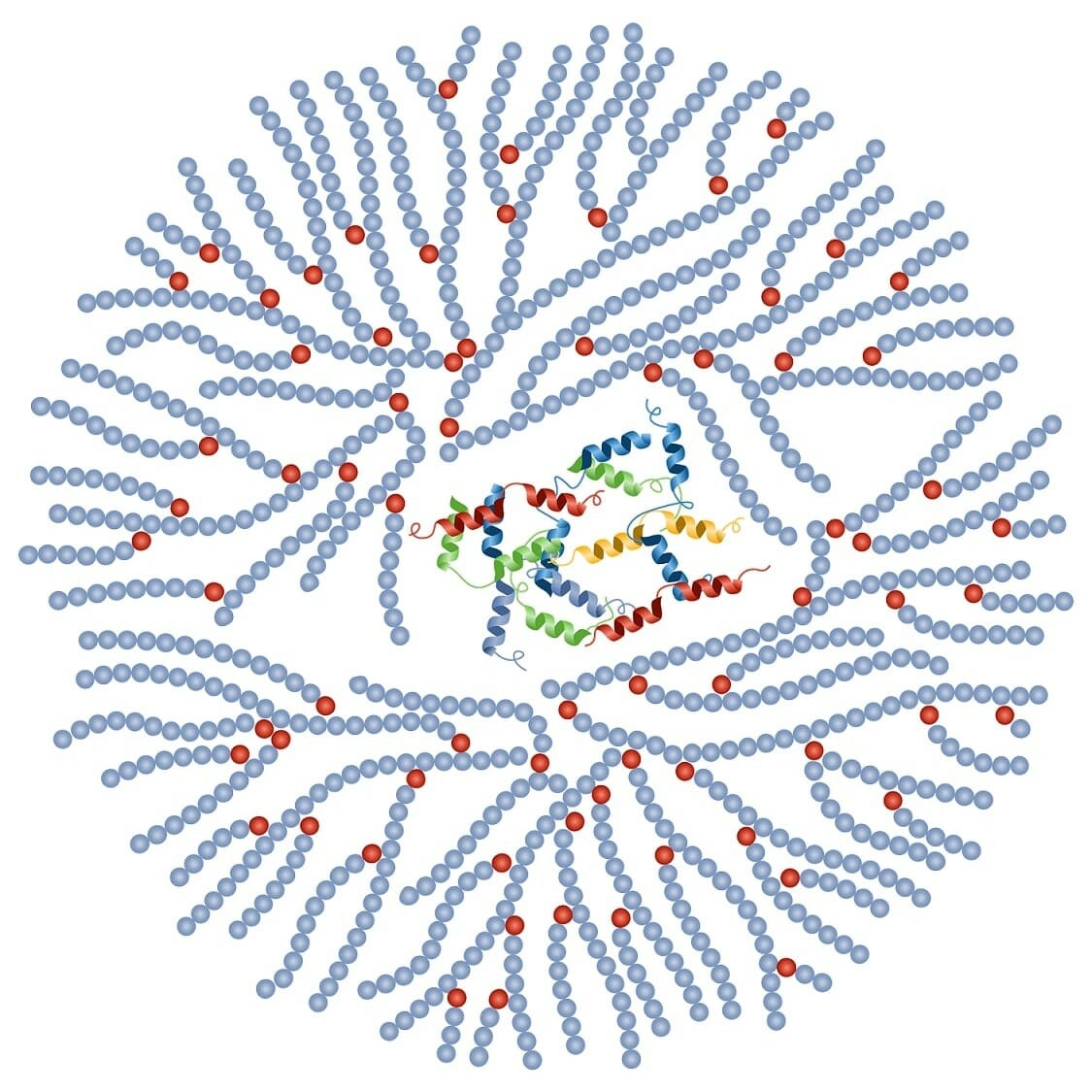
Structure of glycogen:
Glycogen consists of a core protein and is surrounded by 30,000–50,000 glucose units.
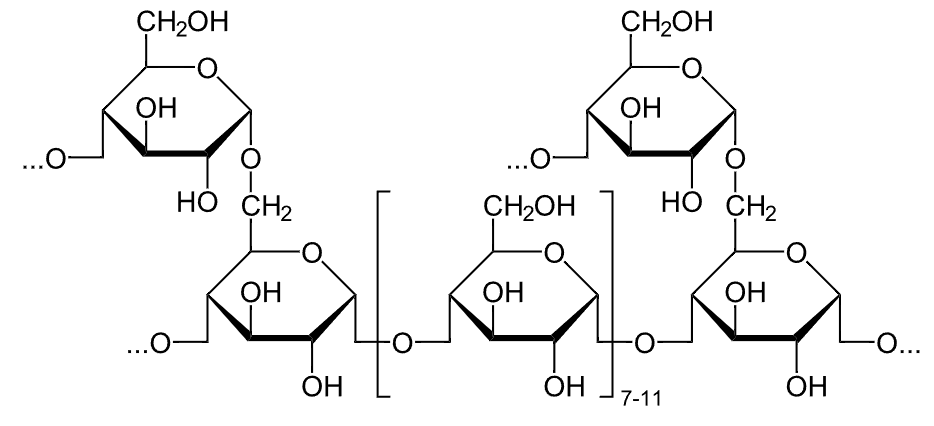
Chemical structure of glycogen
Image: “Glykogen” by NEUROtiker. License: Public Domain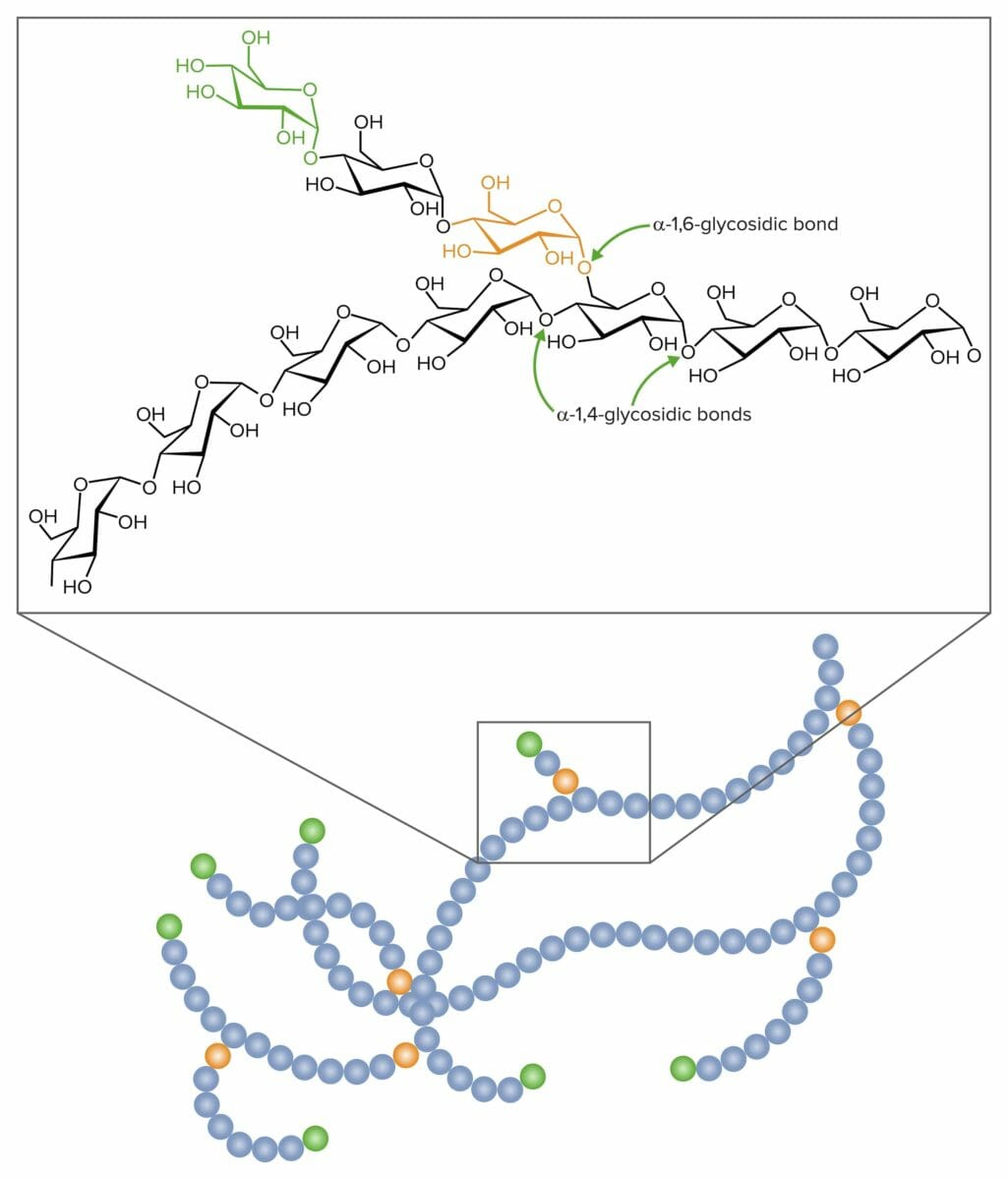
Glycosidic links in the formation of glycogen:
𝛂-1,4-glycosidic and 𝛂-1,6-glycosidic bonds
There are 2 main metabolic pathways of glycogen:
Isomerization of glucose-6-phosphate Glucose-6-phosphate An ester of glucose with phosphoric acid, made in the course of glucose metabolism by mammalian and other cells. It is a normal constituent of resting muscle and probably is in constant equilibrium with fructose-6-phosphate. Gluconeogenesis to glucose-1-phosphate
Reaction of glucose-1-phosphate with uridine triphosphate Uridine triphosphate Uridine 5′-(tetrahydrogen triphosphate). A uracil nucleotide containing three phosphate groups esterified to the sugar moiety. Purine and Pyrimidine Metabolism (UTP) to form an activated form of glucose Glucose A primary source of energy for living organisms. It is naturally occurring and is found in fruits and other parts of plants in its free state. It is used therapeutically in fluid and nutrient replacement. Lactose Intolerance:
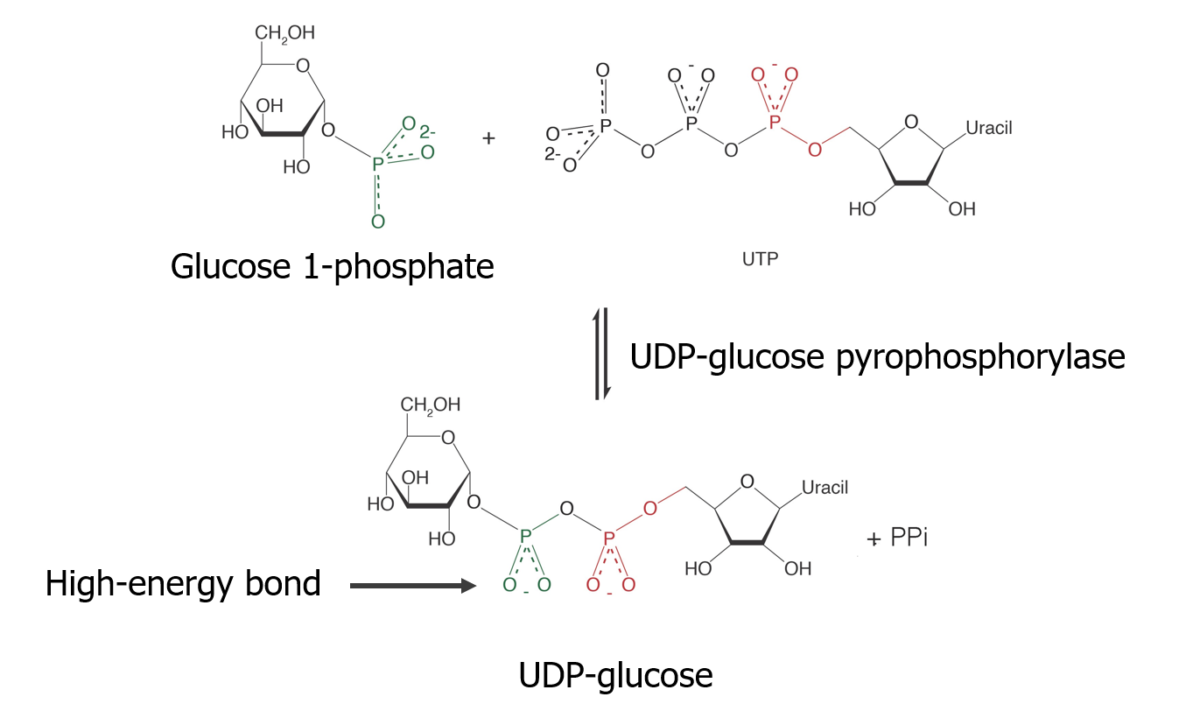
Glycogen synthesis — Making the substrate:
Formation of UDP-glucose which is an active form in glycogenesis
Formation of glycosidic bond Glycosidic bond Basics of Carbohydrates
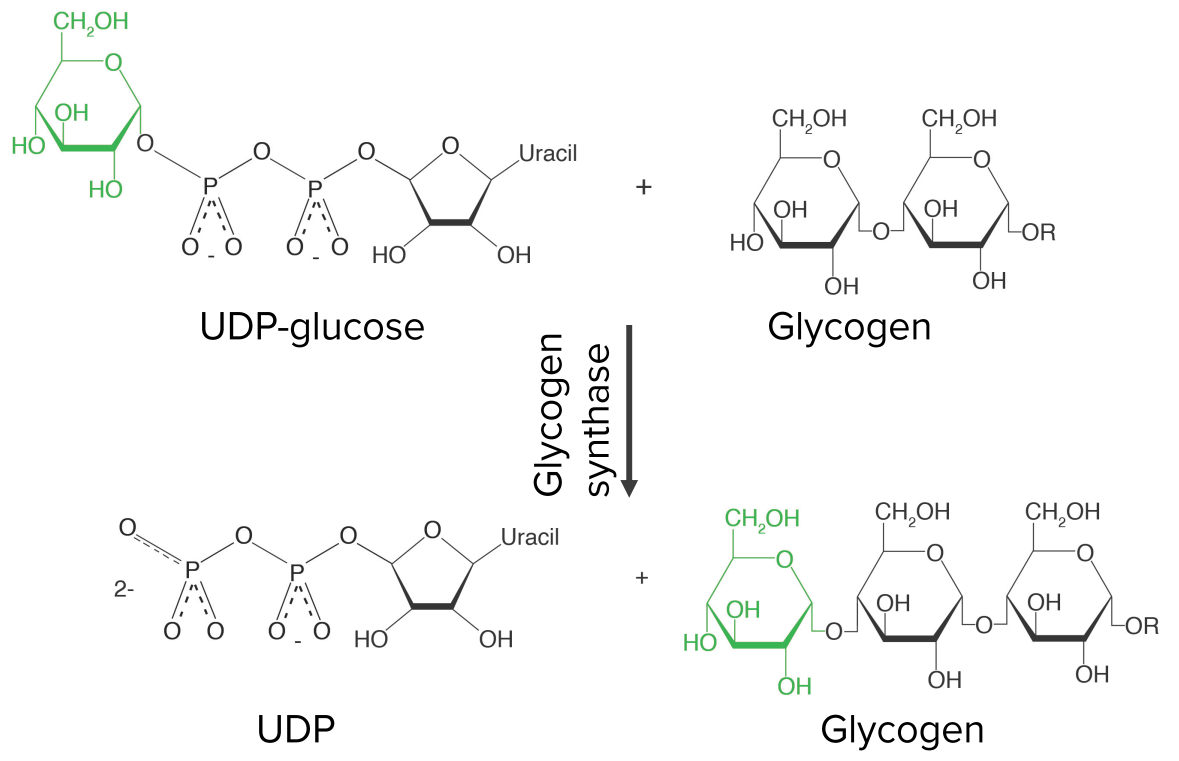
Growing the glycogen chain—step 3 of glycogenesis:
Uridine diphosphate glucose (UDP-glucose) is attached to the hydroxyl group of an already-existing glycogen chain, releasing UDP in the process. The reaction is catalyzed by glycogen synthase, the key regulatory enzyme of glycogenesis.
Branching of glycogen:
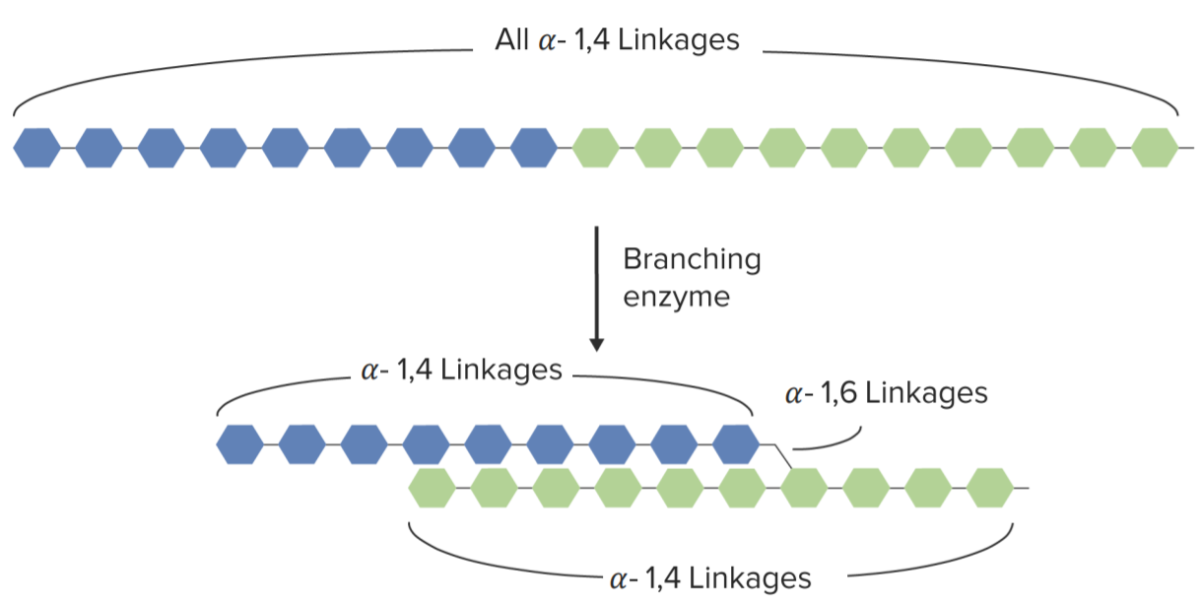
Branching of glycogen chain mediated by the branching enzyme
Image by Lecturio.Breakdown of glycogen to release energy in between meals or after the depletion of glucose Glucose A primary source of energy for living organisms. It is naturally occurring and is found in fruits and other parts of plants in its free state. It is used therapeutically in fluid and nutrient replacement. Lactose Intolerance
Breakdown of glycogen into glucose-1-phosphate:
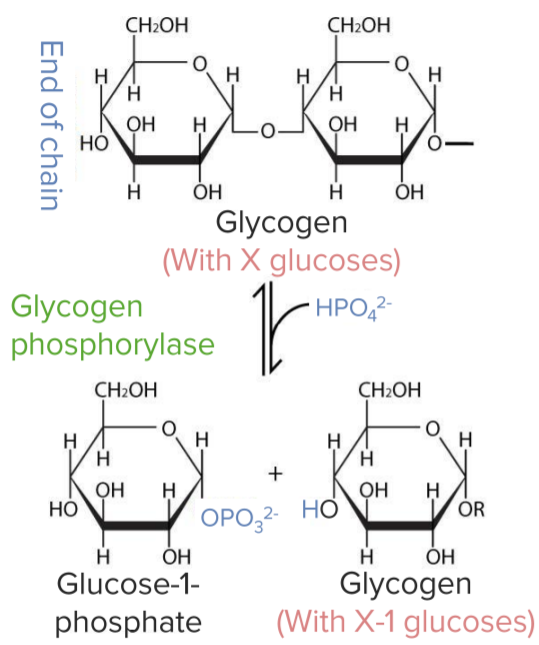
Glycogen breakdown:
Conversion of glycogen to glucose-1-phosphate by the enzyme glycogen phosphorylase
Removal of alpha-1,6-glycosidic bonds (branches):
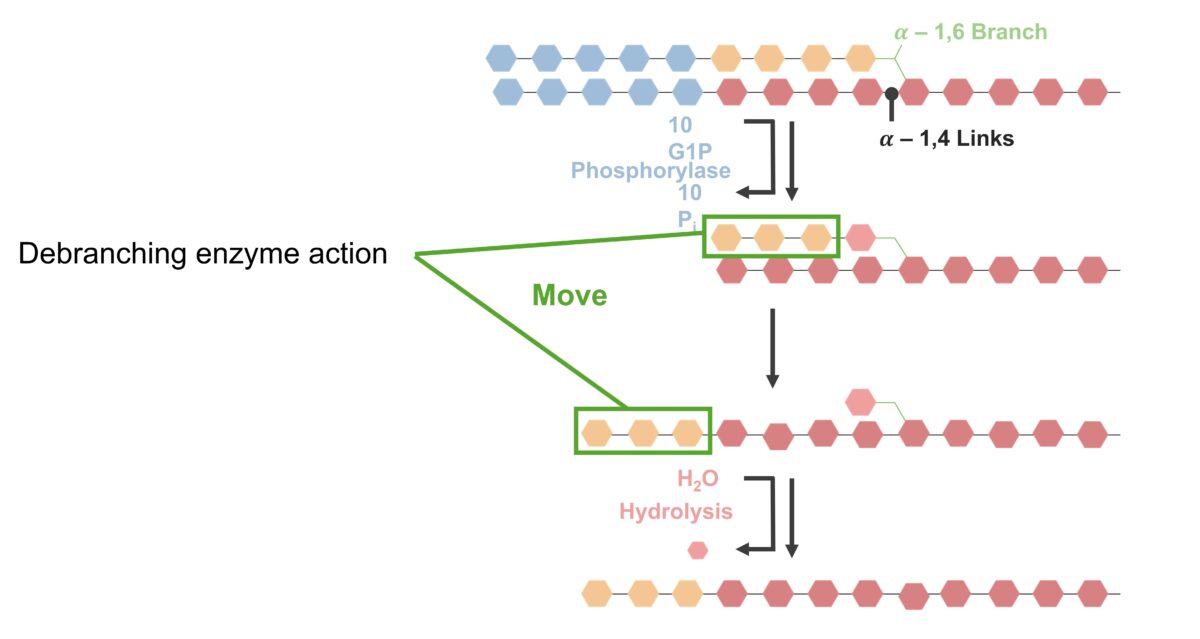
Glycogenolysis (breakdown of bonds and debranching):
Alpha-1,4-glycosidic bonds are broken from the terminal end, catalyzed by phosphorylase. The bonds between glucose residues (blue) are hydrolyzed, releasing glucose-1-phosphate. Phosphorylase hydrolyzes alpha-1,4-glycosidic bonds until only 4 glucose residues (orange) are left before the alpha-1,6 branch. The debranching enzyme (with a transferase and glucosidase activity) then acts on the remaining linked residues. Three of the 4 glucose residues (orange) are removed, leaving 1 molecule. The 3-glucose molecule chain (from the branch) is reattached to the nonreducing end of the linear chain, catalyzed by glucan transferase. The single remaining molecule (in the branch) is removed by the alpha-1,6 glucosidase by hydrolysis, releasing the glucose-1-phosphate. Phosphorylase/debranching process repeats to generate glucose-1-phosphate for energy use.
Conversion of the released glucose-1-phosphate to glucose-6-phosphate Glucose-6-phosphate An ester of glucose with phosphoric acid, made in the course of glucose metabolism by mammalian and other cells. It is a normal constituent of resting muscle and probably is in constant equilibrium with fructose-6-phosphate. Gluconeogenesis:
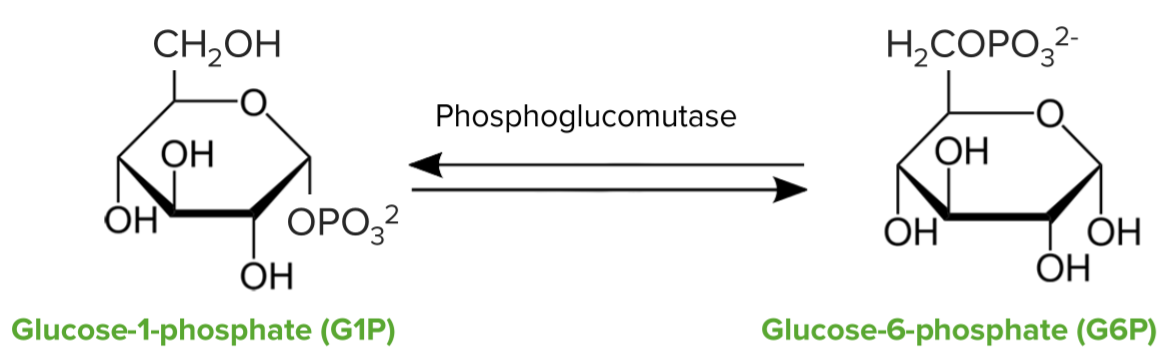
Glycogen breakdown—glycogen phosphorylase:
Conversion of glucose-1-phosphate to glucose-6-phosphate by the enzyme phosphoglucomutase
Glucagon Glucagon A 29-amino acid pancreatic peptide derived from proglucagon which is also the precursor of intestinal glucagon-like peptides. Glucagon is secreted by pancreatic alpha cells and plays an important role in regulation of blood glucose concentration, ketone metabolism, and several other biochemical and physiological processes. Gastrointestinal Secretions and epinephrine Epinephrine The active sympathomimetic hormone from the adrenal medulla. It stimulates both the alpha- and beta- adrenergic systems, causes systemic vasoconstriction and gastrointestinal relaxation, stimulates the heart, and dilates bronchi and cerebral vessels. Sympathomimetic Drugs activate adenylate cyclase in the cell membrane Cell Membrane A cell membrane (also known as the plasma membrane or plasmalemma) is a biological membrane that separates the cell contents from the outside environment. A cell membrane is composed of a phospholipid bilayer and proteins that function to protect cellular DNA and mediate the exchange of ions and molecules. The Cell: Cell Membrane via G proteins Proteins Linear polypeptides that are synthesized on ribosomes and may be further modified, crosslinked, cleaved, or assembled into complex proteins with several subunits. The specific sequence of amino acids determines the shape the polypeptide will take, during protein folding, and the function of the protein. Energy Homeostasis.
| Hormone | Glycogenesis | Glycogenolysis | Serum Glucose Glucose A primary source of energy for living organisms. It is naturally occurring and is found in fruits and other parts of plants in its free state. It is used therapeutically in fluid and nutrient replacement. Lactose Intolerance |
|---|---|---|---|
| Insulin Insulin Insulin is a peptide hormone that is produced by the beta cells of the pancreas. Insulin plays a role in metabolic functions such as glucose uptake, glycolysis, glycogenesis, lipogenesis, and protein synthesis. Exogenous insulin may be needed for individuals with diabetes mellitus, in whom there is a deficiency in endogenous insulin or increased insulin resistance. Insulin | Activation | Inhibition | Decreases |
| Glucagon Glucagon A 29-amino acid pancreatic peptide derived from proglucagon which is also the precursor of intestinal glucagon-like peptides. Glucagon is secreted by pancreatic alpha cells and plays an important role in regulation of blood glucose concentration, ketone metabolism, and several other biochemical and physiological processes. Gastrointestinal Secretions | Inhibition | Activation | Increases |
| Epinephrine Epinephrine The active sympathomimetic hormone from the adrenal medulla. It stimulates both the alpha- and beta- adrenergic systems, causes systemic vasoconstriction and gastrointestinal relaxation, stimulates the heart, and dilates bronchi and cerebral vessels. Sympathomimetic Drugs | Inhibition | Activation | Increases |
| Effectors | Glycogenolysis | Glycogenesis |
|---|---|---|
| cAMP cAMP An adenine nucleotide containing one phosphate group which is esterified to both the 3′- and 5′-positions of the sugar moiety. It is a second messenger and a key intracellular regulator, functioning as a mediator of activity for a number of hormones, including epinephrine, glucagon, and acth. Phosphodiesterase Inhibitors | ↑ | ↓ |
| PK | ↑ | ↓ |
| AMP (muscle) | ↑ | ↓ |
| Calcium Calcium A basic element found in nearly all tissues. It is a member of the alkaline earth family of metals with the atomic symbol ca, atomic number 20, and atomic weight 40. Calcium is the most abundant mineral in the body and combines with phosphorus to form calcium phosphate in the bones and teeth. It is essential for the normal functioning of nerves and muscles and plays a role in blood coagulation (as factor IV) and in many enzymatic processes. Electrolytes/muscle contraction | ↑ | ↓ |
| Hormones Hormones Hormones are messenger molecules that are synthesized in one part of the body and move through the bloodstream to exert specific regulatory effects on another part of the body. Hormones play critical roles in coordinating cellular activities throughout the body in response to the constant changes in both the internal and external environments. Hormones: Overview and Types | Glycogenolysis | Glycogenesis |
|---|---|---|
| Insulin Insulin Insulin is a peptide hormone that is produced by the beta cells of the pancreas. Insulin plays a role in metabolic functions such as glucose uptake, glycolysis, glycogenesis, lipogenesis, and protein synthesis. Exogenous insulin may be needed for individuals with diabetes mellitus, in whom there is a deficiency in endogenous insulin or increased insulin resistance. Insulin | ↓ | ↑ |
| Glucagon Glucagon A 29-amino acid pancreatic peptide derived from proglucagon which is also the precursor of intestinal glucagon-like peptides. Glucagon is secreted by pancreatic alpha cells and plays an important role in regulation of blood glucose concentration, ketone metabolism, and several other biochemical and physiological processes. Gastrointestinal Secretions | ↑ | ↓ |
| Epinephrine Epinephrine The active sympathomimetic hormone from the adrenal medulla. It stimulates both the alpha- and beta- adrenergic systems, causes systemic vasoconstriction and gastrointestinal relaxation, stimulates the heart, and dilates bronchi and cerebral vessels. Sympathomimetic Drugs | ↑ | ↓ |
| Regulatory Enzyme | Glycogenolysis | Glycogenesis |
|---|---|---|
| Glycogen synthase | Activated (enzyme is phosphorylated) | Inactivated |
| Glycogen synthase | Inactivated | Activated (enzyme is dephosphorylated) |
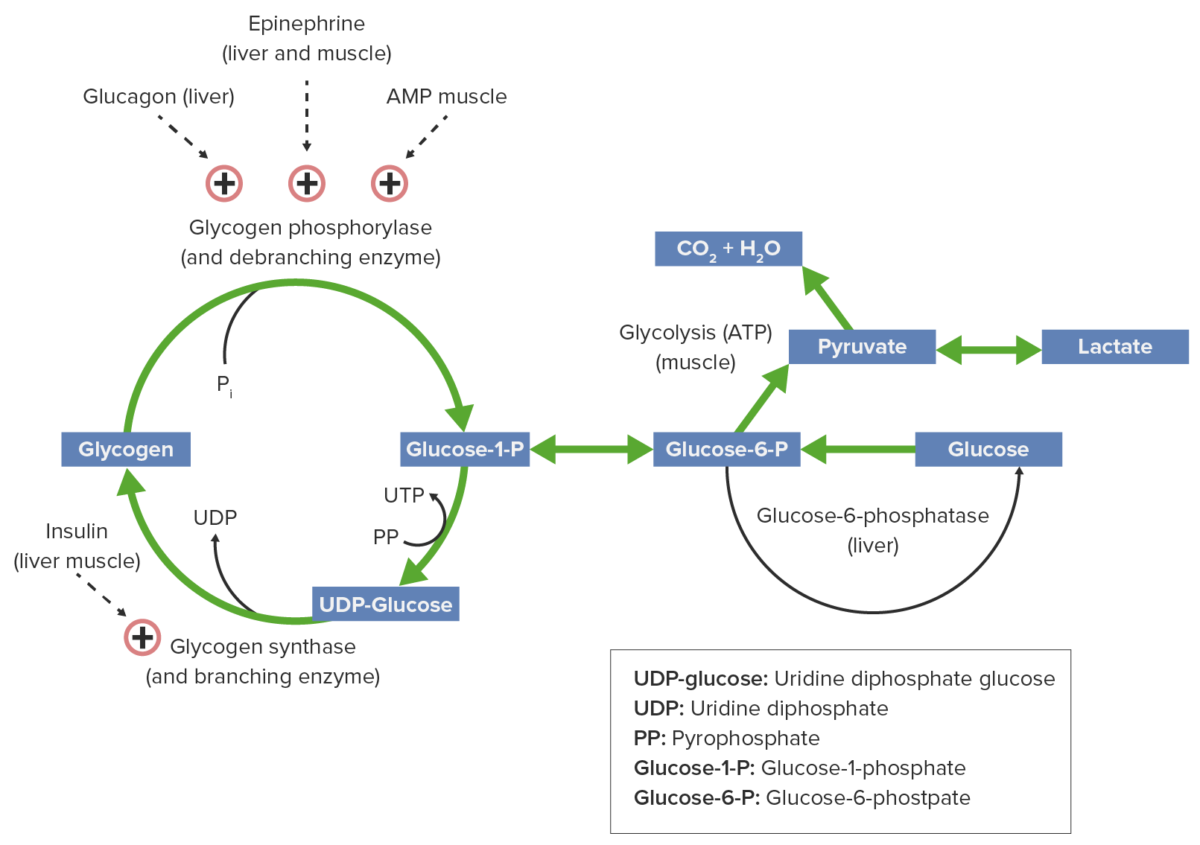
Glycogen metabolism and regulatory factors:
Epinephrine, glucagon, and AMP activate glycogen phosphorylase, thus glycogenolysis is promoted, producing glucose for energy consumption. Insulin activates glycogen synthase, facilitating glycogen buildup.
Glycogen storage disease Glycogen storage disease A group of inherited metabolic disorders involving the enzymes responsible for the synthesis and degradation of glycogen. In some patients, prominent liver involvement is presented. In others, more generalized storage of glycogen occurs, sometimes with prominent cardiac involvement. Benign Liver Tumors: a group of inherited disorders characterized by abnormalities in glycogen metabolism, resulting in an abnormal accumulation of glycogen in the tissues.
| Type | Diseases | Enzyme Deficiency | Clinical Features |
|---|---|---|---|
| 0 | Lewis disease | Glycogen synthase |
|
| Ⅰ | Von Gierke disease | Glucose-6-phosphatase Glucose-6-phosphatase An enzyme that catalyzes the conversion of d-glucose 6-phosphate and water to d-glucose and orthophosphate. Gluconeogenesis |
|
| Ⅱ | Pompe disease | Lysosomal α-1,4 and α-1,6 glucosidase (acid maltase Maltase Digestion and Absorption of Carbohydrates) |
|
| Ⅲ | Forbes-Cori disease | Debranching enzyme (amylo-1,6-glucosidase) |
|
| Ⅳ | Andersen disease Andersen disease An autosomal recessive metabolic disorder due to a deficiency in expression of glycogen branching enzyme 1 (alpha-1, 4-glucan-6-alpha-glucosyltransferase), resulting in an accumulation of abnormal glycogen with long outer branches. Clinical features are muscle hypotonia and cirrhosis. Death from liver disease usually occurs before age 2. Glycogen Storage Disorders | 1,4-α-glucan branching enzyme |
|
| Ⅴ | McArdle disease | Muscle phosphorylase |
|
| Ⅵ | Hers disease | Liver Liver The liver is the largest gland in the human body. The liver is found in the superior right quadrant of the abdomen and weighs approximately 1.5 kilograms. Its main functions are detoxification, metabolism, nutrient storage (e.g., iron and vitamins), synthesis of coagulation factors, formation of bile, filtration, and storage of blood. Liver: Anatomy phosphorylase |
|
| Ⅶ | Tarui disease Tarui disease An autosomal recessive glycogen storage disease in which there is deficient expression of 6-phosphofructose 1-kinase in muscle resulting in abnormal deposition of glycogen in muscle tissue. These patients have severe congenital muscular dystrophy and are exercise intolerant. Glycogen Storage Disorders | Muscle phosphofructokinase (PFK) |
|
| Ⅷ | Hepatic phosphorylase kinase deficiency Phosphorylase kinase deficiency Glycogen Storage Disorders | Liver Liver The liver is the largest gland in the human body. The liver is found in the superior right quadrant of the abdomen and weighs approximately 1.5 kilograms. Its main functions are detoxification, metabolism, nutrient storage (e.g., iron and vitamins), synthesis of coagulation factors, formation of bile, filtration, and storage of blood. Liver: Anatomy phosphorylase kinase |
|
| Ⅸ | Phosphorylase kinase deficiency Phosphorylase kinase deficiency Glycogen Storage Disorders | Liver Liver The liver is the largest gland in the human body. The liver is found in the superior right quadrant of the abdomen and weighs approximately 1.5 kilograms. Its main functions are detoxification, metabolism, nutrient storage (e.g., iron and vitamins), synthesis of coagulation factors, formation of bile, filtration, and storage of blood. Liver: Anatomy and muscle phosphorylase kinase |
|
| Ⅹ | PGAM deficiency | Phosphoglycerate mutase Phosphoglycerate mutase An enzyme that catalyzes the conversion of 2-phospho-d-glycerate to 3-phospho-d-glycerate. Glycolysis |
|
| ⅩⅠ | Lactate dehydrogenase Lactate Dehydrogenase Osteosarcoma A deficiency | Lactate dehydrogenase Lactate Dehydrogenase Osteosarcoma A |
|
| XII | Aldolase Aldolase Becker Muscular Dystrophy A deficiency | Aldolase Aldolase Becker Muscular Dystrophy A |
|
| XIII | Beta-enolase deficiency Beta-enolase deficiency Glycogen Storage Disorders (muscle) | Beta-enolase |
|
| XIV | Phosphoglucomutase I deficiency (muscle) | Phosphoglucomutase I |
|
| XV | Glycogenin I deficiency (muscle) | Glycogenin I |
|
| Fanconi-Bickel syndrome | GLUT2 GLUT2 A glucose transport facilitator that is expressed primarily in pancreatic beta cells; liver; and kidneys. It may function as a glucose sensor to regulate insulin release and glucose homeostasis. Digestion and Absorption |
|