Interferon (IFN) is a cytokine with antiviral Antiviral Antivirals for Hepatitis B properties (it interferes with viral infections Infections Invasion of the host organism by microorganisms or their toxins or by parasites that can cause pathological conditions or diseases. Chronic Granulomatous Disease) and various roles in immunoregulation. The different types are type I IFN (IFN-ɑ and IFN-β), type II IFN (IFN-ɣ), and type III IFN (IFN-ƛ). Type I IFNs have been extensively studied; these proteins Proteins Linear polypeptides that are synthesized on ribosomes and may be further modified, crosslinked, cleaved, or assembled into complex proteins with several subunits. The specific sequence of amino acids determines the shape the polypeptide will take, during protein folding, and the function of the protein. Energy Homeostasis bind BIND Hyperbilirubinemia of the Newborn to cell-surface receptors Receptors Receptors are proteins located either on the surface of or within a cell that can bind to signaling molecules known as ligands (e.g., hormones) and cause some type of response within the cell. Receptors when triggered by a viral infection. After stimulation, pathways are activated to produce proteins Proteins Linear polypeptides that are synthesized on ribosomes and may be further modified, crosslinked, cleaved, or assembled into complex proteins with several subunits. The specific sequence of amino acids determines the shape the polypeptide will take, during protein folding, and the function of the protein. Energy Homeostasis (e.g., ribonuclease) that inhibit viral replication. An antiviral Antiviral Antivirals for Hepatitis B state is created in both infected and uninfected cells. Type I IFN also has antitumor properties. The antiviral Antiviral Antivirals for Hepatitis B activity of type II IFN (IFN-ɣ) is not as potent as that of type I, but IFN- ɣ is crucial in macrophage activation Macrophage activation The process of altering the morphology and functional activity of macrophages so that they become avidly phagocytic. It is initiated by lymphokines, such as the macrophage activation factor (maf) and the macrophage migration-inhibitory factor (mmif), immune complexes, C3b, and various peptides, polysaccharides, and immunologic adjuvants. IL-12 Receptor Deficiency. The recently discovered IFN-ƛ is noted to have activity against intestinal viruses Viruses Minute infectious agents whose genomes are composed of DNA or RNA, but not both. They are characterized by a lack of independent metabolism and the inability to replicate outside living host cells. Virology. With a wide range of biologic effects, interferons are used in therapy for malignancies, infections Infections Invasion of the host organism by microorganisms or their toxins or by parasites that can cause pathological conditions or diseases. Chronic Granulomatous Disease, and other immune-related conditions (e.g., multiple sclerosis Sclerosis A pathological process consisting of hardening or fibrosis of an anatomical structure, often a vessel or a nerve. Wilms Tumor).
Last updated: May 17, 2024
Interferons are a group of proteins Proteins Linear polypeptides that are synthesized on ribosomes and may be further modified, crosslinked, cleaved, or assembled into complex proteins with several subunits. The specific sequence of amino acids determines the shape the polypeptide will take, during protein folding, and the function of the protein. Energy Homeostasis belonging to a class of signaling molecules Signaling molecules Second Messengers known as cytokines Cytokines Non-antibody proteins secreted by inflammatory leukocytes and some non-leukocytic cells, that act as intercellular mediators. They differ from classical hormones in that they are produced by a number of tissue or cell types rather than by specialized glands. They generally act locally in a paracrine or autocrine rather than endocrine manner. Adaptive Immune Response and are released by a variety of cells during the inflammatory response.
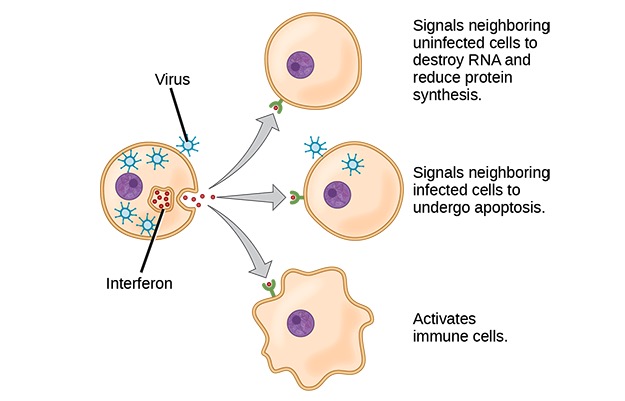
Interferons:
Interferons are cytokines that are released by cells infected with a virus, leukocytes, and other immune cells. To limit the infection, responses of cells to interferon include inhibition of protein synthesis, activation of immune cells, and induction of apoptosis.
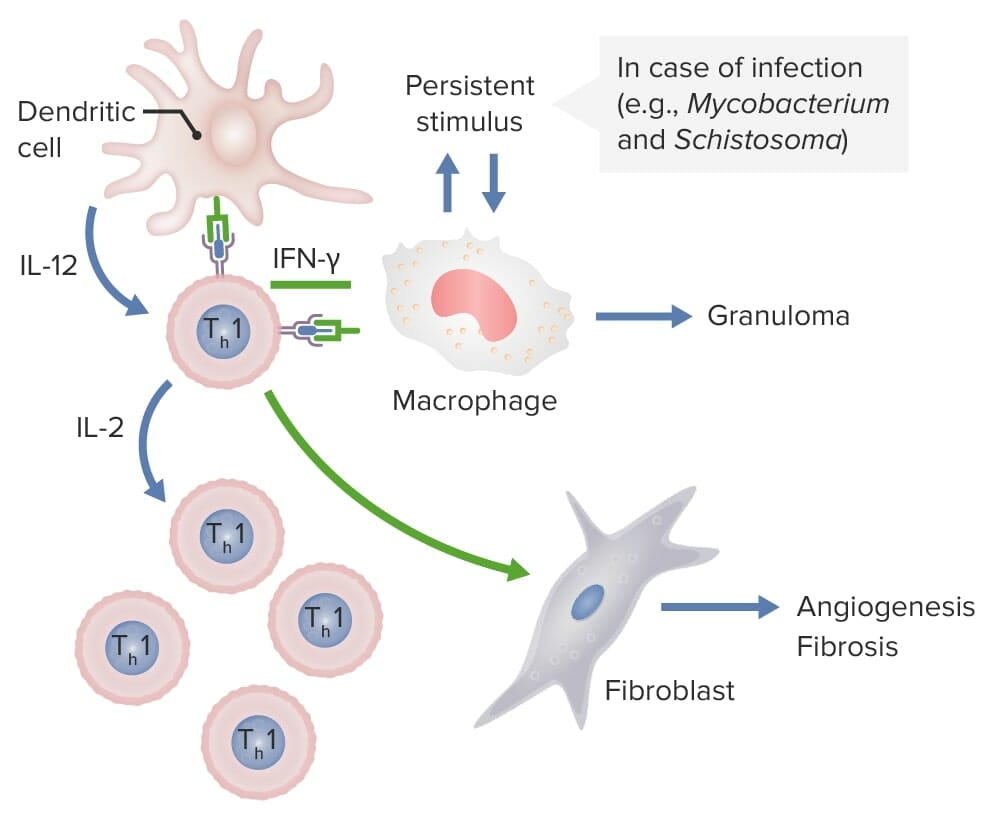
Dendritic cells release IL-12, which activates CD4 Th1 cells. These Th1 cells produce IL-2, stimulating production of more Th1 T-cell subsets. Th1 cells also release IFN-γ, which activates macrophages and activates fibroblasts to cause angiogenesis and fibrosis. If these macrophages are persistently stimulated by pathogens, such as Mycobacterium and Schistosoma, granulomas are formed.
Image by Lecturio.| Other designation | Chromosomal location | Cell of origin | |
|---|---|---|---|
| IFN-ɑ | Intron-A | 9p22 | Leukocytes Leukocytes White blood cells. These include granular leukocytes (basophils; eosinophils; and neutrophils) as well as non-granular leukocytes (lymphocytes and monocytes). White Myeloid Cells: Histology |
| IFN-β | IFN-b2 | 9p21 | Fibroblasts Fibroblasts Connective tissue cells which secrete an extracellular matrix rich in collagen and other macromolecules. Sarcoidosis |
| IFN-ɣ | Macrophage activating factor: immune-interferon | 12q14 | Lymphocytes Lymphocytes Lymphocytes are heterogeneous WBCs involved in immune response. Lymphocytes develop from the bone marrow, starting from hematopoietic stem cells (HSCs) and progressing to common lymphoid progenitors (CLPs). B and T lymphocytes and natural killer (NK) cells arise from the lineage. Lymphocytes: Histology, macrophages Macrophages The relatively long-lived phagocytic cell of mammalian tissues that are derived from blood monocytes. Main types are peritoneal macrophages; alveolar macrophages; histiocytes; kupffer cells of the liver; and osteoclasts. They may further differentiate within chronic inflammatory lesions to epithelioid cells or may fuse to form foreign body giant cells or langhans giant cells. Innate Immunity: Phagocytes and Antigen Presentation, NK cells NK cells A specialized subset of T-lymphocytes that exhibit features of innate immunity similar to that of natural killer cells. They are reactive to glycolipids presented in the context of the major histocompatibility complex (MHC) class I-like molecule, CD1D antigen. Lymphocytes: Histology, dendritic cells Dendritic cells Specialized cells of the hematopoietic system that have branch-like extensions. They are found throughout the lymphatic system, and in non-lymphoid tissues such as skin and the epithelia of the intestinal, respiratory, and reproductive tracts. They trap and process antigens, and present them to T-cells, thereby stimulating cell-mediated immunity. They are different from the non-hematopoietic follicular dendritic cells, which have a similar morphology and immune system function, but with respect to humoral immunity (antibody production). Skin: Structure and Functions |
| IFN-ƛ | IL-28A, IL-28B, IL-29, IFNA14 | 19q13.13 | Epithelial cells |
| Interferon | Condition(s) treated |
|---|---|
| Interferon-α |
|
| Interferon-β | Multiple sclerosis Sclerosis A pathological process consisting of hardening or fibrosis of an anatomical structure, often a vessel or a nerve. Wilms Tumor |
| Interferon-γ |
|