Neisseria is a genus of bacteria Bacteria Bacteria are prokaryotic single-celled microorganisms that are metabolically active and divide by binary fission. Some of these organisms play a significant role in the pathogenesis of diseases. Bacteriology commonly present on mucosal surfaces. Several species exist, but only 2 are pathogenic to humans: N. gonorrhoeae and N. meningitidis. Neisseria species are non-motile, gram-negative diplococci most commonly isolated on modified Thayer-Martin (MTM) agar. These pathogens have many virulence factors Virulence factors Those components of an organism that determine its capacity to cause disease but are not required for its viability per se. Two classes have been characterized: toxins, biological and surface adhesion molecules that affect the ability of the microorganism to invade and colonize a host. Haemophilus, including fimbriae Fimbriae Thin, hairlike appendages, 1 to 20 microns in length and often occurring in large numbers, present on the cells of gram-negative bacteria, particularly enterobacteriaceae and Neisseria. Unlike flagella, they do not possess motility, but being protein (pilin) in nature, they possess antigenic and hemagglutinating properties. They are of medical importance because some fimbriae mediate the attachment of bacteria to cells via adhesins. Bacterial fimbriae refer to common pili, to be distinguished from the preferred use of 'pili'. Escherichia coli, lipooligosaccharide envelope Envelope Bilayer lipid membrane acquired by viral particles during viral morphogenesis. Although the lipids of the viral envelope are host derived, various virus-encoded integral membrane proteins, i.e. Viral envelope proteins are incorporated there. Virology proteins Proteins Linear polypeptides that are synthesized on ribosomes and may be further modified, crosslinked, cleaved, or assembled into complex proteins with several subunits. The specific sequence of amino acids determines the shape the polypeptide will take, during protein folding, and the function of the protein. Energy Homeostasis, a polysaccharide capsule Capsule An envelope of loose gel surrounding a bacterial cell which is associated with the virulence of pathogenic bacteria. Some capsules have a well-defined border, whereas others form a slime layer that trails off into the medium. Most capsules consist of relatively simple polysaccharides but there are some bacteria whose capsules are made of polypeptides. Bacteroides (unique to N. meningitidis), and IgA IgA Represents 15-20% of the human serum immunoglobulins, mostly as the 4-chain polymer in humans or dimer in other mammals. Secretory iga is the main immunoglobulin in secretions. Immunoglobulins: Types and Functions protease Protease Enzyme of the human immunodeficiency virus that is required for post-translational cleavage of gag and gag-pol precursor polyproteins into functional products needed for viral assembly. HIV protease is an aspartic protease encoded by the amino terminus of the pol gene. HIV Infection and AIDS. Gonococcal infections Infections Invasion of the host organism by microorganisms or their toxins or by parasites that can cause pathological conditions or diseases. Chronic Granulomatous Disease are sexually or perinatally transmitted and include gonorrhea Gonorrhea Gonorrhea is a sexually transmitted infection (STI) caused by the gram-negative bacteria Neisseria gonorrhoeae (N. gonorrhoeae). Gonorrhea may be asymptomatic but commonly manifests as cervicitis or urethritis with less common presentations such as proctitis, conjunctivitis, or pharyngitis. Gonorrhea, pelvic inflammatory disease Pelvic inflammatory disease Pelvic inflammatory disease (PID) is defined as a polymicrobial infection of the upper female reproductive system. The disease can affect the uterus, fallopian tubes, ovaries, and adjacent structures. Pelvic inflammatory disease is closely linked with sexually transmitted diseases, most commonly caused by Chlamydia trachomatis, Neisseria gonorrhoeae, and Gardnerella vaginalis. Pelvic Inflammatory Disease, septic arthritis Arthritis Acute or chronic inflammation of joints. Osteoarthritis, and neonatal conjunctivitis Conjunctivitis Conjunctivitis is a common inflammation of the bulbar and/or palpebral conjunctiva. It can be classified into infectious (mostly viral) and noninfectious conjunctivitis, which includes allergic causes. Patients commonly present with red eyes, increased tearing, burning, foreign body sensation, and photophobia. Conjunctivitis. Meningococcal infections Infections Invasion of the host organism by microorganisms or their toxins or by parasites that can cause pathological conditions or diseases. Chronic Granulomatous Disease are transmitted via respiratory and oral secretions. They most commonly cause meningococcemia Meningococcemia Terminal Complement Pathway Deficiency with petechial hemorrhages Petechial Hemorrhages Brown-Séquard Syndrome and meningitis Meningitis Meningitis is inflammation of the meninges, the protective membranes of the brain, and spinal cord. The causes of meningitis are varied, with the most common being bacterial or viral infection. The classic presentation of meningitis is a triad of fever, altered mental status, and nuchal rigidity. Meningitis.
Last updated: Dec 30, 2024
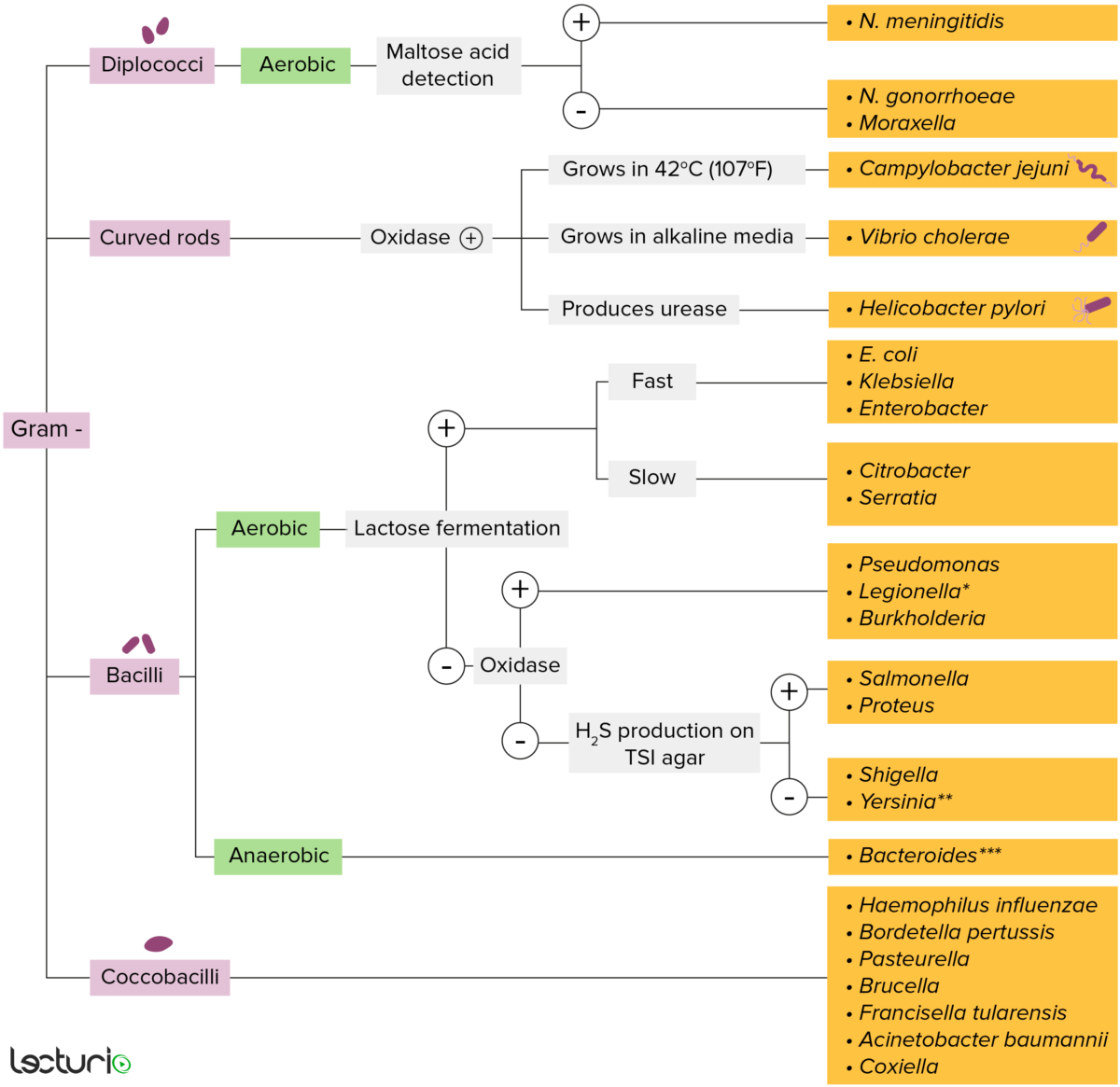
Gram-negative bacteria:
Most bacteria can be classified according to a lab procedure called Gram staining.
Bacteria with cell walls that have a thin layer of peptidoglycan do not retain the crystal violet stain utilized in Gram staining. These bacteria do, however, retain the safranin counterstain and thus appear as pinkish-red on the stain, making them gram negative. These bacteria can be further classified according to morphology (diplococci, curved rods, bacilli, and coccobacilli) and their ability to grow in the presence of oxygen (aerobic versus anaerobic). The bacteria can be more narrowly identified by growing them on specific media (triple sugar iron (TSI) agar) where their enzymes can be identified (urease, oxidase) and their ability to ferment lactose can be tested.
* Stains poorly on Gram stain
** Pleomorphic rod/coccobacillus
*** Require special transport media
| N. meningitidis | N. gonorrhoeae |
|---|---|
| Encapsulated Encapsulated Klebsiella (polysaccharide capsule Capsule An envelope of loose gel surrounding a bacterial cell which is associated with the virulence of pathogenic bacteria. Some capsules have a well-defined border, whereas others form a slime layer that trails off into the medium. Most capsules consist of relatively simple polysaccharides but there are some bacteria whose capsules are made of polypeptides. Bacteroides) | Not capsulated |
| Ferments maltose and glucose Glucose A primary source of energy for living organisms. It is naturally occurring and is found in fruits and other parts of plants in its free state. It is used therapeutically in fluid and nutrient replacement. Lactose Intolerance | Ferments glucose Glucose A primary source of energy for living organisms. It is naturally occurring and is found in fruits and other parts of plants in its free state. It is used therapeutically in fluid and nutrient replacement. Lactose Intolerance only |
| Colonizes nasopharynx Nasopharynx The top portion of the pharynx situated posterior to the nose and superior to the soft palate. The nasopharynx is the posterior extension of the nasal cavities and has a respiratory function. Pharynx: Anatomy | Colonizes genital mucosa |
| Rarely has plasmids Plasmids Extrachromosomal, usually circular DNA molecules that are self-replicating and transferable from one organism to another. They are found in a variety of bacterial, archaeal, fungal, algal, and plant species. They are used in genetic engineering as cloning vectors. DNA Types and Structure | Most have plasmids Plasmids Extrachromosomal, usually circular DNA molecules that are self-replicating and transferable from one organism to another. They are found in a variety of bacterial, archaeal, fungal, algal, and plant species. They are used in genetic engineering as cloning vectors. DNA Types and Structure |
General virulence factors Virulence factors Those components of an organism that determine its capacity to cause disease but are not required for its viability per se. Two classes have been characterized: toxins, biological and surface adhesion molecules that affect the ability of the microorganism to invade and colonize a host. Haemophilus:
Mechanisms to evade immune recognition Immune Recognition Yaws, Bejel, and Pinta:
Mechanisms of antimicrobial resistance Resistance Physiologically, the opposition to flow of air caused by the forces of friction. As a part of pulmonary function testing, it is the ratio of driving pressure to the rate of air flow. Ventilation: Mechanics of Breathing:
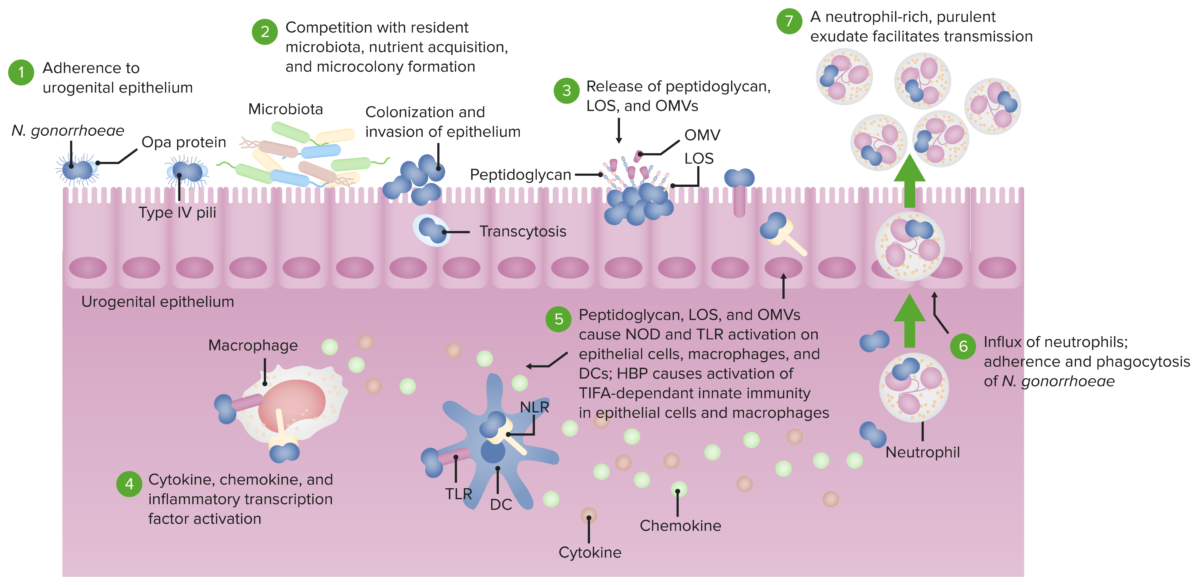
Pathogenesis of N. gonorrhoeae.
The bacterium adheres to the mucosal epithelium (1) where it competes with microbiota and colonizes to invade the epithelium (2). Peptidoglycans, LOS, and OMVs are released (3), activating TLR and NOD signaling in epithelial cells, macrophages, and dendritic cells (4). This leads to the production of cytokines and chemokines (5) which brings about a neutrophil-rich purulent exudate and phagocytosis of the harmful bacteria (6). Since N. gonorrhoeae has defense mechanisms that resist being killed, the bacteria-laden neutrophils can also act as agents of transmission to another host (7).
Selected abbreviation key:
LOS: lipooligosaccharide
OMV: outer membrane vesicles
TLR: toll-like receptor
NOD: nucleotide-binding oligomerization domain-containing protein
| Type of presentation | Clinical features |
|---|---|
| Gonorrhea Gonorrhea Gonorrhea is a sexually transmitted infection (STI) caused by the gram-negative bacteria Neisseria gonorrhoeae (N. gonorrhoeae). Gonorrhea may be asymptomatic but commonly manifests as cervicitis or urethritis with less common presentations such as proctitis, conjunctivitis, or pharyngitis. Gonorrhea (acute gonococcal infection; commonly referred to as “the clap”) |
|
| Pelvic inflammatory disease Pelvic inflammatory disease Pelvic inflammatory disease (PID) is defined as a polymicrobial infection of the upper female reproductive system. The disease can affect the uterus, fallopian tubes, ovaries, and adjacent structures. Pelvic inflammatory disease is closely linked with sexually transmitted diseases, most commonly caused by Chlamydia trachomatis, Neisseria gonorrhoeae, and Gardnerella vaginalis. Pelvic Inflammatory Disease ( PID PID Pelvic inflammatory disease (PID) is defined as a polymicrobial infection of the upper female reproductive system. The disease can affect the uterus, fallopian tubes, ovaries, and adjacent structures. Pelvic inflammatory disease is closely linked with sexually transmitted diseases, most commonly caused by Chlamydia trachomatis, Neisseria gonorrhoeae, and gardnerella vaginalis. Pelvic Inflammatory Disease) |
|
| Neonatal conjunctivitis Conjunctivitis Conjunctivitis is a common inflammation of the bulbar and/or palpebral conjunctiva. It can be classified into infectious (mostly viral) and noninfectious conjunctivitis, which includes allergic causes. Patients commonly present with red eyes, increased tearing, burning, foreign body sensation, and photophobia. Conjunctivitis (gonococcal ophthalmia neonatorum Ophthalmia Neonatorum Acute conjunctival inflammation in the newborn, usually caused by maternal gonococcal infection. The causative agent is Neisseria gonorrhoeae. The baby’s eyes are contaminated during passage through the birth canal. Gonorrhea) |
|
| Disseminated gonococcal infection |
|
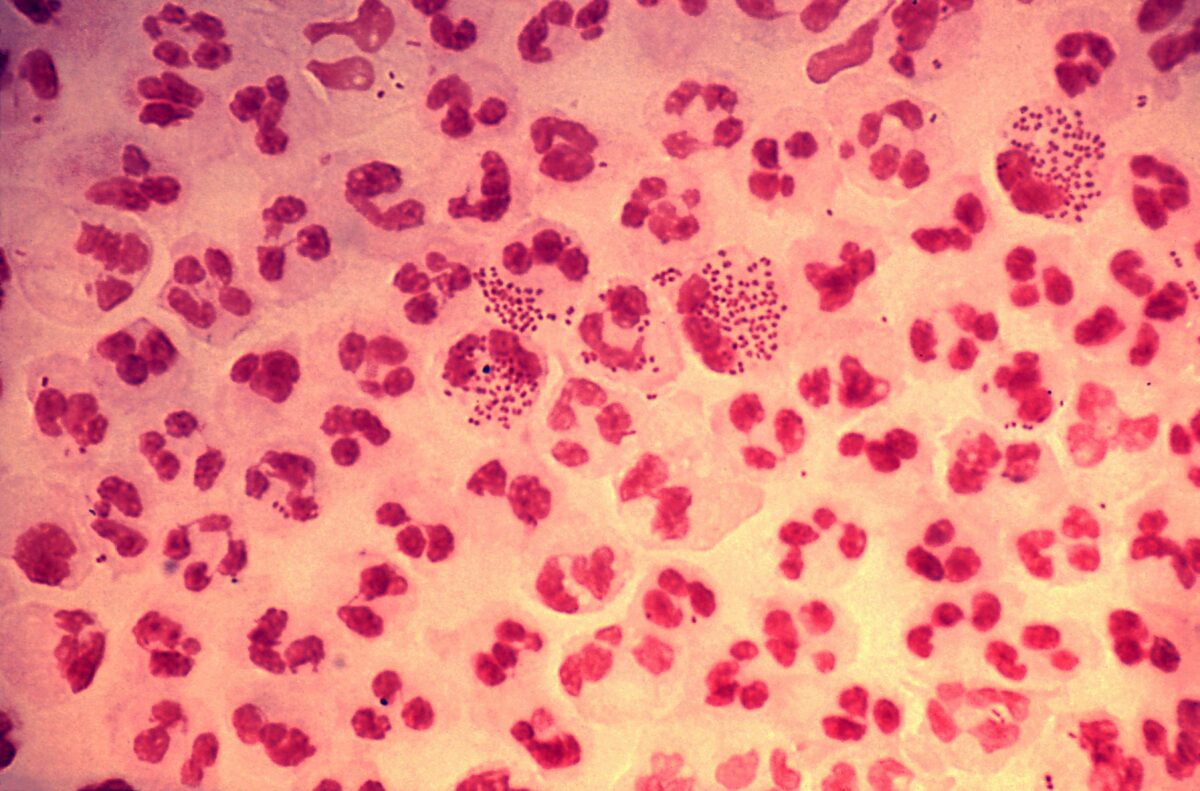
N. gonorrhoeae are gram-negative diplococci that provoke a marked neutrophilic response, as seen in this image, with several neutrophils containing many phagocytized bacteria.
Image: “Gonorrhea Neisseria gonorrhoeae” by Joe Miller. License: CC0Virulence factors Virulence factors Those components of an organism that determine its capacity to cause disease but are not required for its viability per se. Two classes have been characterized: toxins, biological and surface adhesion molecules that affect the ability of the microorganism to invade and colonize a host. Haemophilus:
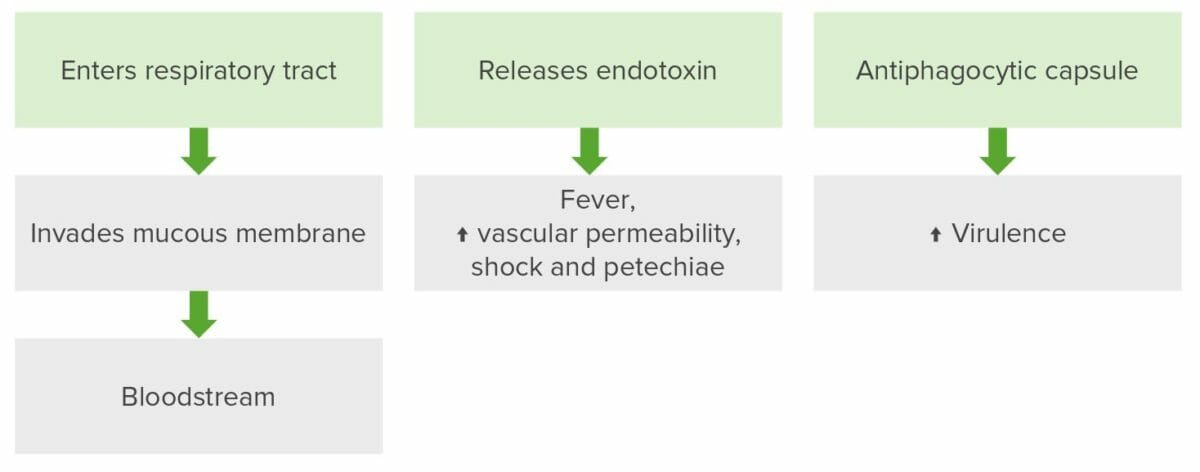
Neisseria meningitidis pathogenesis
The bacteria enters the respiratory system to invade mucous membranes, entering the bloodstream. In the blood, proliferation occurs and the pathogen releases endotoxin which causes fever, increased vascular permeability, shock, and petechiae. The antiphagocytic capsule enables the pathogen to evade destruction by the immune system.
Pathogenesis:
| Type of presentation | Clinical features |
|---|---|
| Meningitis Meningitis Meningitis is inflammation of the meninges, the protective membranes of the brain, and spinal cord. The causes of meningitis are varied, with the most common being bacterial or viral infection. The classic presentation of meningitis is a triad of fever, altered mental status, and nuchal rigidity. Meningitis |
|
| Meningococcemia Meningococcemia Terminal Complement Pathway Deficiency (meningococcal septicemia) |
Acute
meningococcemia
Meningococcemia
Terminal Complement Pathway Deficiency:
Chronic meningococcemia Meningococcemia Terminal Complement Pathway Deficiency:
|
| Waterhouse-Friderichse syndrome |
|
| Other presentations |
|
Prevention is done by quadrivalent meningococcal polysaccharide conjugate vaccine Vaccine Suspensions of killed or attenuated microorganisms (bacteria, viruses, fungi, protozoa), antigenic proteins, synthetic constructs, or other bio-molecular derivatives, administered for the prevention, amelioration, or treatment of infectious and other diseases. Vaccination: