Endocarditis is an inflammatory disease involving the inner lining ( endocardium Endocardium The innermost layer of the heart, comprised of endothelial cells. Heart: Anatomy) of the heart, most commonly affecting the cardiac valves. Both infectious and noninfectious etiologies lead to vegetations on the valve leaflets. Patients Patients Individuals participating in the health care system for the purpose of receiving therapeutic, diagnostic, or preventive procedures. Clinician–Patient Relationship may present with nonspecific symptoms such as fever Fever Fever is defined as a measured body temperature of at least 38°C (100.4°F). Fever is caused by circulating endogenous and/or exogenous pyrogens that increase levels of prostaglandin E2 in the hypothalamus. Fever is commonly associated with chills, rigors, sweating, and flushing of the skin. Fever and fatigue Fatigue The state of weariness following a period of exertion, mental or physical, characterized by a decreased capacity for work and reduced efficiency to respond to stimuli. Fibromyalgia. Important clinical exam findings include a new or changed heart murmur and common extra-cardiac signs, such as Osler nodes, Janeway lesions, splinter hemorrhages, and Roth spots. The diagnosis is based on clinical findings, blood cultures, and echocardiography Echocardiography Ultrasonic recording of the size, motion, and composition of the heart and surrounding tissues. The standard approach is transthoracic. Tricuspid Valve Atresia (TVA) showing valvular vegetations. Management includes intravenous antibiotics for infectious cases, addressing the underlying etiology for noninfectious cases, and surgical repair when necessary.
Last updated: May 16, 2024
Infective endocarditis:
Noninfective endocarditis:
Infective endocarditis may be caused by numerous organisms; the list below is not exhaustive.
The following are risk factors for IE:
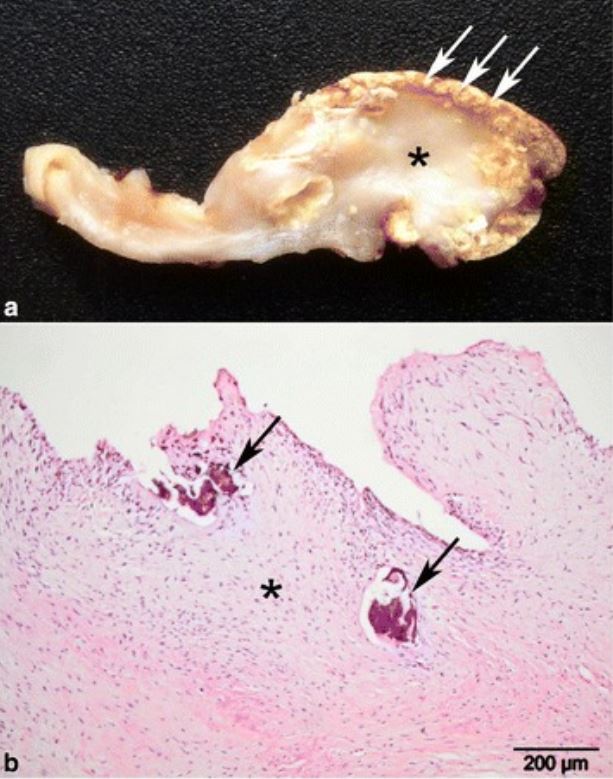
Pathology specimens from a patient with chronic bovine fibrous calcified endocarditis:
a: gross pathology showing extensive fibrosis and thickening of the stroma (*) and widespread superficial calcification (arrows)
b: histopathology of the lesion, showing extensive fibrosis (*) and areas of calcification (arrows)
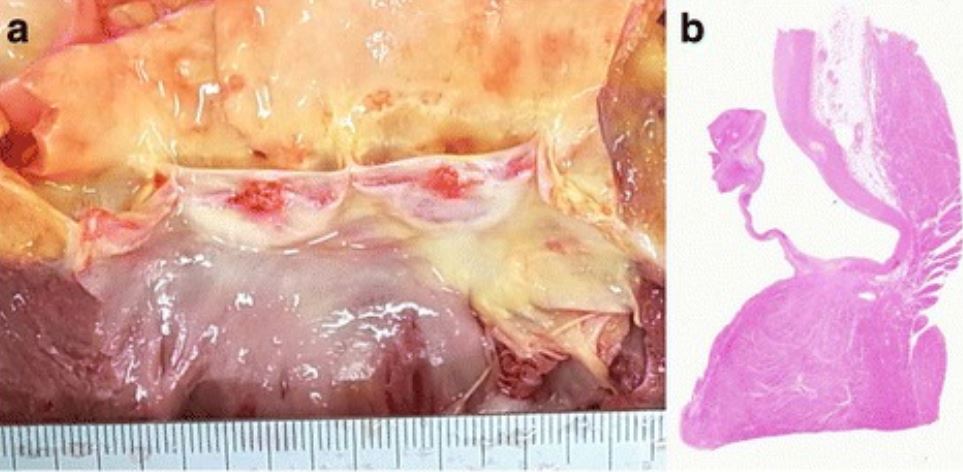
Vegetations of the aortic valve at autopsy:
a: The macroscopic appearance of the aortic valve demonstrates 2 vegetations of 4 mm and 5 mm in diameter.
b: Histologic evaluation shows that the vegetations consist of fibrin without bacterial colonies, consistent with noninfective endocarditis.
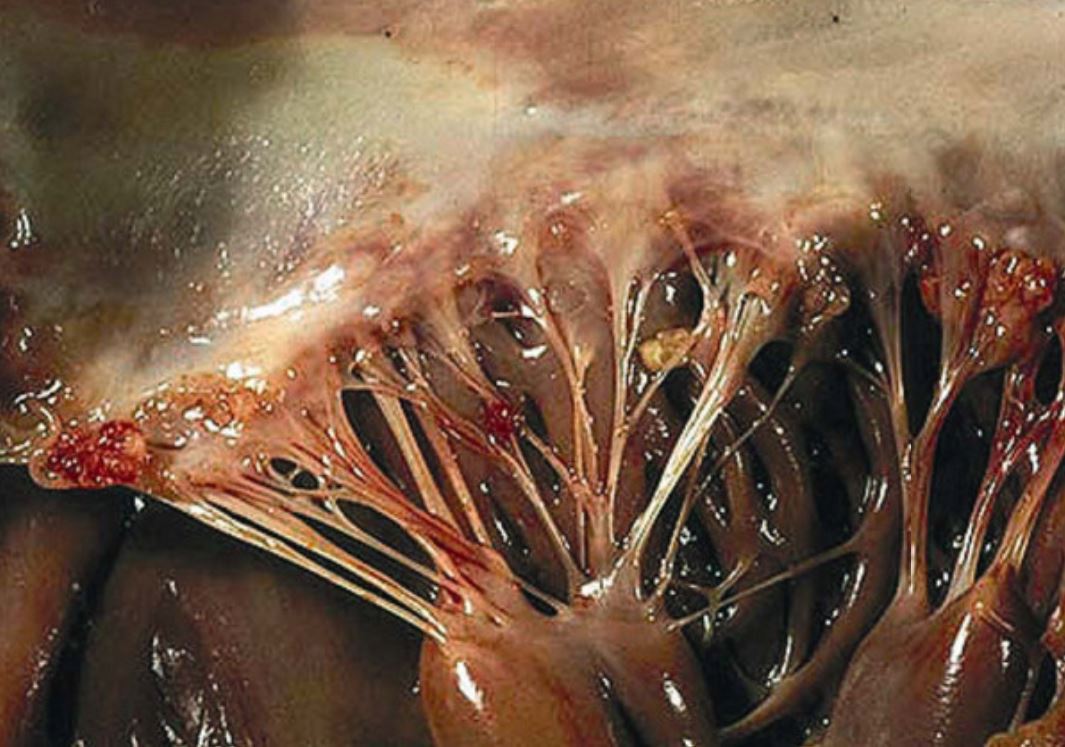
Verrucous vegetations seen in Libman-Sacks endocarditis of the mitral valve:
The sterile vegetations typically have a wart-like morphology. The vegetations can be found near the edge of the leaflets along the line of closure, both on the atrial and ventricular sides of the leaflets, and can even be found on the chordae and the endocardium. In this case, several microthrombi are present on the free edge of the leaflet and on the chordae.
Infective endocarditis can be further classified based on the clinical course, type of valve, and location.
Acute infectious endocarditis:
Subacute infectious endocarditis (endocarditis lenta):
Native valve endocarditis:
Prosthetic valve Prosthetic Valve Soft Tissue Abscess endocarditis:
Left-sided endocarditis:
Right-sided endocarditis (most common in IV drug use):
Presentation and course depend on the etiology, location of vegetations, and severity.
The following are more frequently seen in IE than NIE:
The following are potential findings in IE:
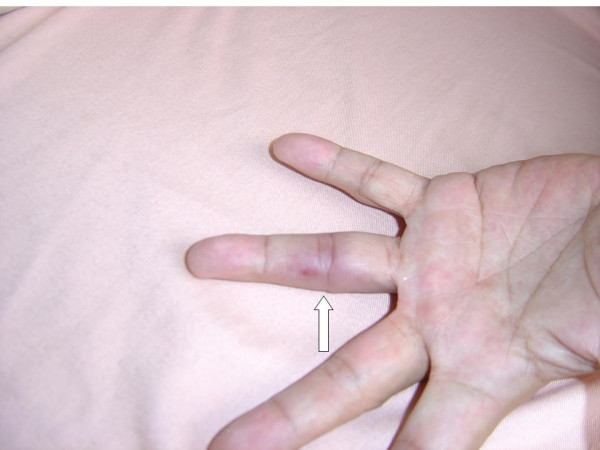
Osler node on the 4th digit of the left hand
Image: “Osler’s node on the fourth digit of the left hand” by Yang ML, Chen YH, Chen TC, Lin WR, Lin CY, Lu PL. License: CC BY 2.0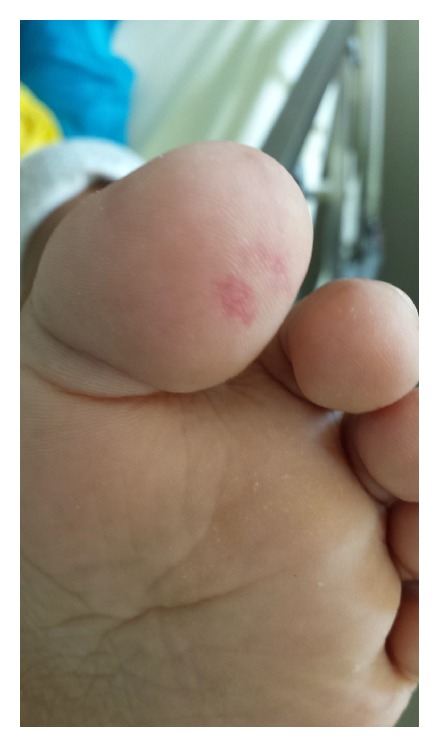
Janeway lesion on the toe in a patient with infective endocarditis
Image: “Janeway lesion on patient’s left toe” by Case Reports in Infectious Diseases. License: CC BY 4.0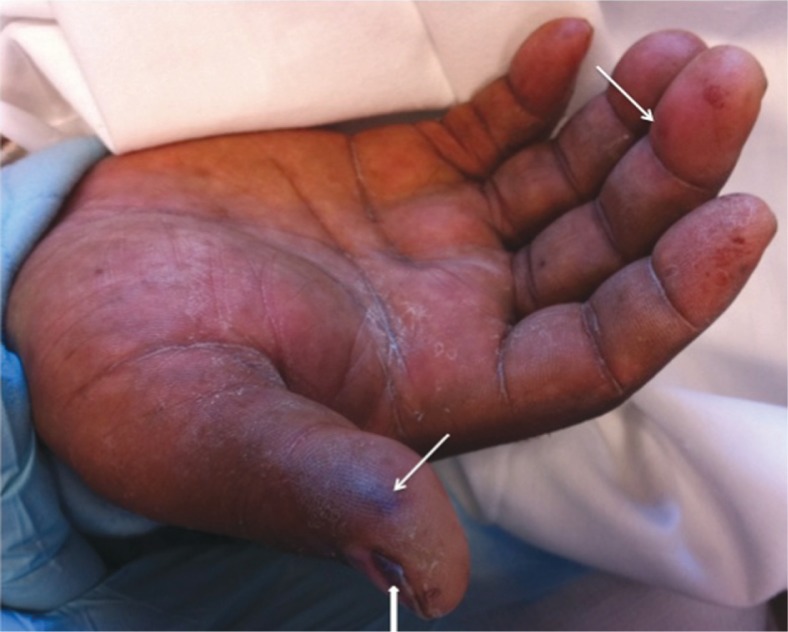
Janeway lesion showing as painless, macular, hemorrhagic, irregularly-shaped lesions on patient’s palm:
Two pronounced lesions are seen at thumb and middle finger. Subungual splinter hemorrhages (arrowhead) are seen at the nail bed of thumb.
Signs of IE can be remembered with the mnemonic “FROM JANE”:
System embolization Embolization A method of hemostasis utilizing various agents such as gelfoam, silastic, metal, glass, or plastic pellets, autologous clot, fat, and muscle as emboli. It has been used in the treatment of spinal cord and intracranial arteriovenous malformations, renal arteriovenous fistulas, gastrointestinal bleeding, epistaxis, hypersplenism, certain highly vascular tumors, traumatic rupture of blood vessels, and control of operative hemorrhage. Gastrointestinal Bleeding may occur in both IE and NIE. Often, an embolic event is the only presenting evidence of NIE.
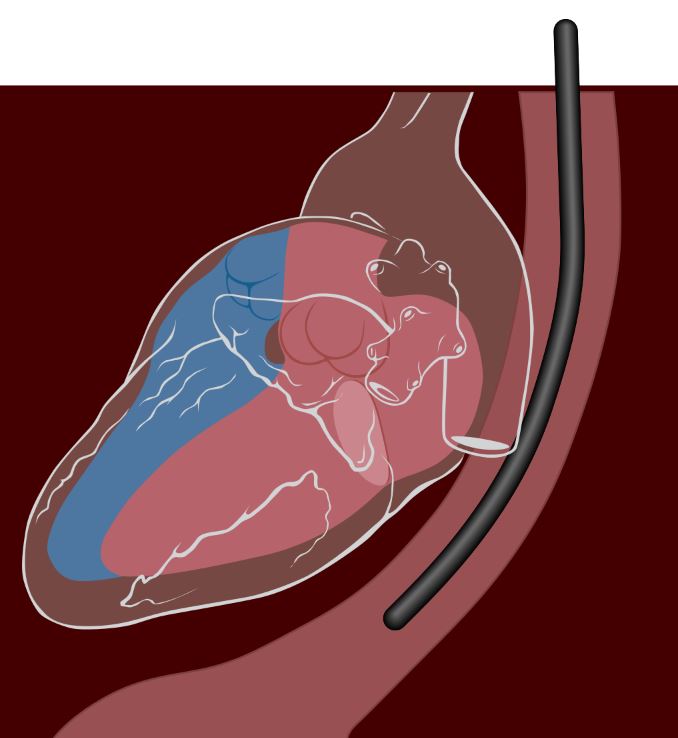
Depiction of transesophageal echocardiography:
Patients are generally sedated for this procedure. The echo transducer is lowered into the esophagus, which places it right next to the left side of the heart.
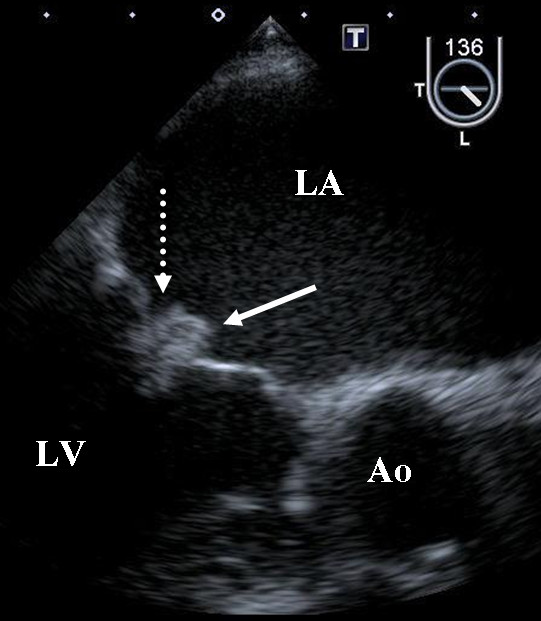
Transesophageal echocardiography images demonstrating vegetations on the mitral valve in a patient with noninfective endocarditis due to malignancy.
The solid arrow is pointing to a vegetation on the anterior leaflet, and the dotted arrow is pointing to a vegetation on the posterior leaflet.
Ao: aorta; LA: left atrium; LV: left ventricle
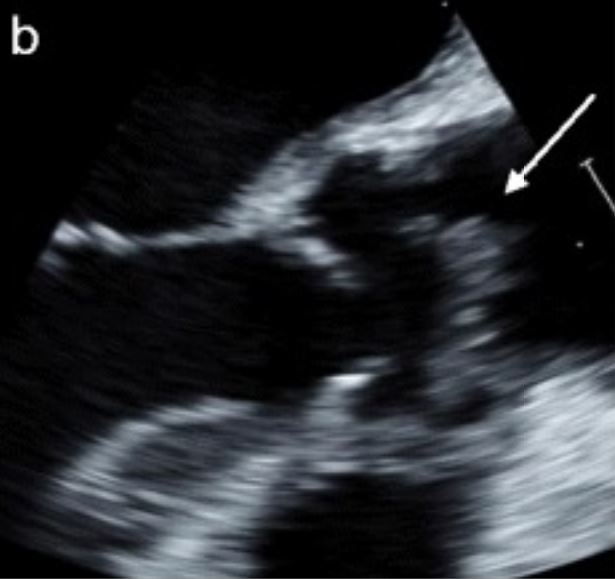
Transesophageal echocardiography image demonstrating a large pedunculated vegetation within the ascending aorta arising from the commissure of the right and noncoronary cusp of the aortic valve with a calcified base consistent with endocarditis
Image: “Lomentospora prolificans endocarditis–case report and literature review” by Kelly M, Stevens R, Konecny P. License: CC BY 4.0, cropped by Lecturio.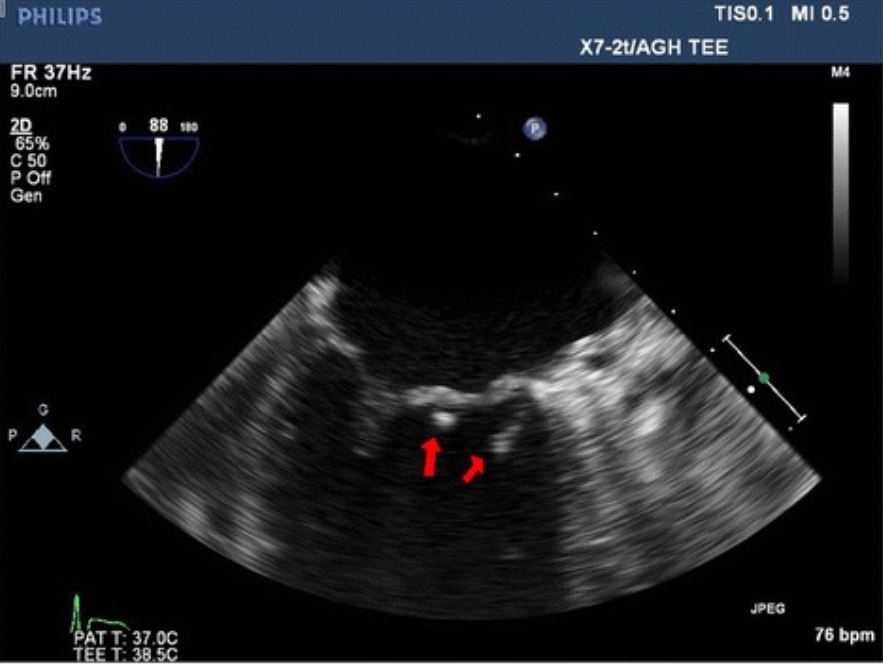
Transesophageal echocardiography image showing 2 vegetations (red arrows) on the mitral valve in a patient with endocarditis
Image: “Pseudomonas mendocina native valve infective endocarditis: a case report” by Journal of Medical Case Reports. License: CC BY 4.0Laboratory findings:
Imaging:
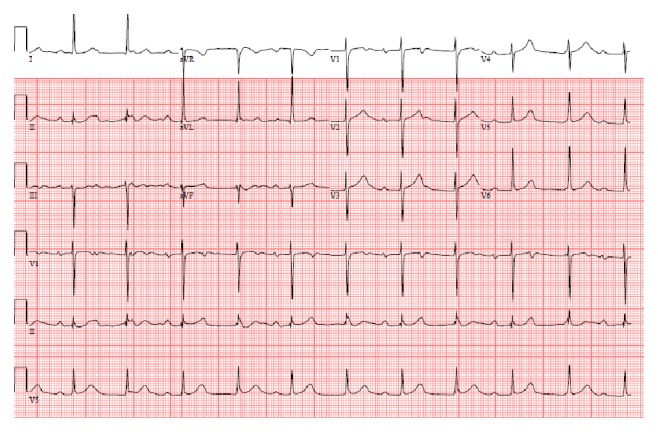
An ECG showing atrioventricular dissociation in a patient with S. viridans endocarditis
Image: “Timing for pacing after acquired conduction disease in the setting of endocarditis” by Brancheau D, Degheim G, Machado C. License: CC BY 3.0The Duke diagnostic criteria is a set of clinical criteria that can aid in the diagnosis of IE.
Prompt initiation of IV antibiotics is necessary if the patient is acutely ill.
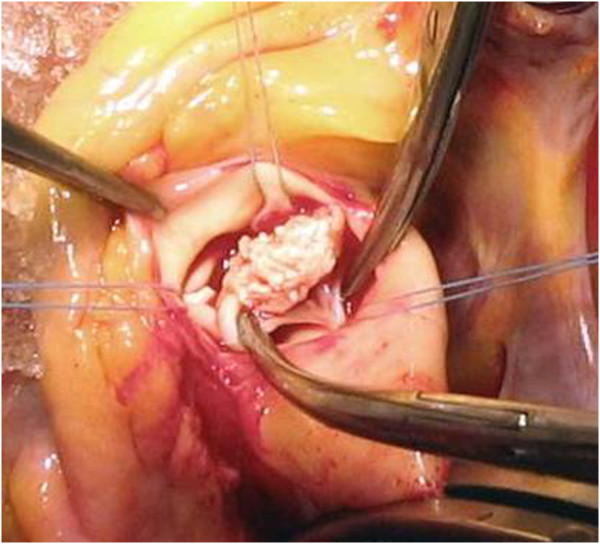
Intraoperative vegetation findings on the aortic valve in a patient with endocarditis
Image: “A rare case of Candida parapsilosis endocarditis in a young healthy woman” by Pelemiš, M., et al. License: CC BY 2.0