Tricuspid valve Tricuspid valve The valve consisting of three cusps situated between the right atrium and right ventricle of the heart. Heart: Anatomy atresia Atresia Hypoplastic Left Heart Syndrome (HLHS) (TVA) is a congenital heart defect (CHD) causing an absent or rudimentary tricuspid valve Tricuspid valve The valve consisting of three cusps situated between the right atrium and right ventricle of the heart. Heart: Anatomy (TV) associated with an interatrial or ventricular septal defect Ventricular Septal Defect Tetralogy of Fallot. Patients Patients Individuals participating in the health care system for the purpose of receiving therapeutic, diagnostic, or preventive procedures. Clinician–Patient Relationship present with cyanosis Cyanosis A bluish or purplish discoloration of the skin and mucous membranes due to an increase in the amount of deoxygenated hemoglobin in the blood or a structural defect in the hemoglobin molecule. Pulmonary Examination at birth due to the shunting of deoxygenated blood from the right atrium (RA) into the left atrium (LA). Diagnosis can be made in utero or confirmed after birth with an echocardiogram Echocardiogram Transposition of the Great Arteries. Definitive management involves a staged surgical procedure beginning in the neonatal period.
Last updated: Jan 9, 2025
Tricuspid valve Tricuspid valve The valve consisting of three cusps situated between the right atrium and right ventricle of the heart. Heart: Anatomy atresia Atresia Hypoplastic Left Heart Syndrome (HLHS) (TVA) is a congenital heart defect (CHD) featuring the complete absence or agenesis Agenesis Teratogenic Birth Defects of the tricuspid valve Tricuspid valve The valve consisting of three cusps situated between the right atrium and right ventricle of the heart. Heart: Anatomy (TV) resulting in a lack of communication Communication The exchange or transmission of ideas, attitudes, or beliefs between individuals or groups. Decision-making Capacity and Legal Competence between the right atrium (RA) and the right ventricle (RV). Tricuspid valve Tricuspid valve The valve consisting of three cusps situated between the right atrium and right ventricle of the heart. Heart: Anatomy atresia Atresia Hypoplastic Left Heart Syndrome (HLHS) (TVA) is considered a cyanotic congenital heart disease (CCHD) as most patients Patients Individuals participating in the health care system for the purpose of receiving therapeutic, diagnostic, or preventive procedures. Clinician–Patient Relationship present with cyanosis Cyanosis A bluish or purplish discoloration of the skin and mucous membranes due to an increase in the amount of deoxygenated hemoglobin in the blood or a structural defect in the hemoglobin molecule. Pulmonary Examination at birth.
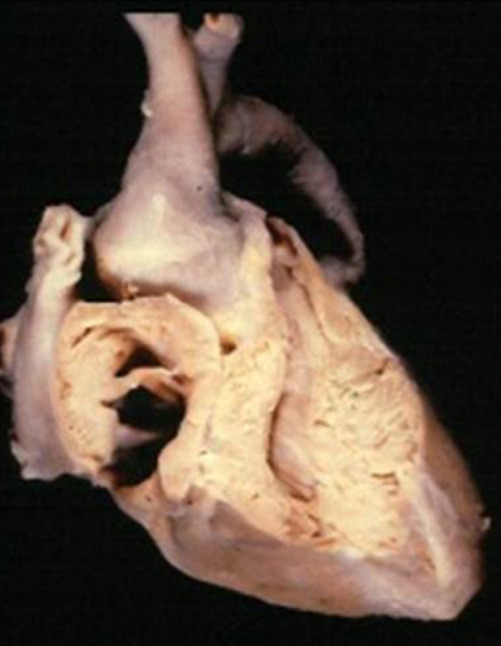
Tricuspid atresia:
Note the thick muscular floor base of the RA in place of the TV in the cadaver sample. The image indicates no communication between the RA and the RV.
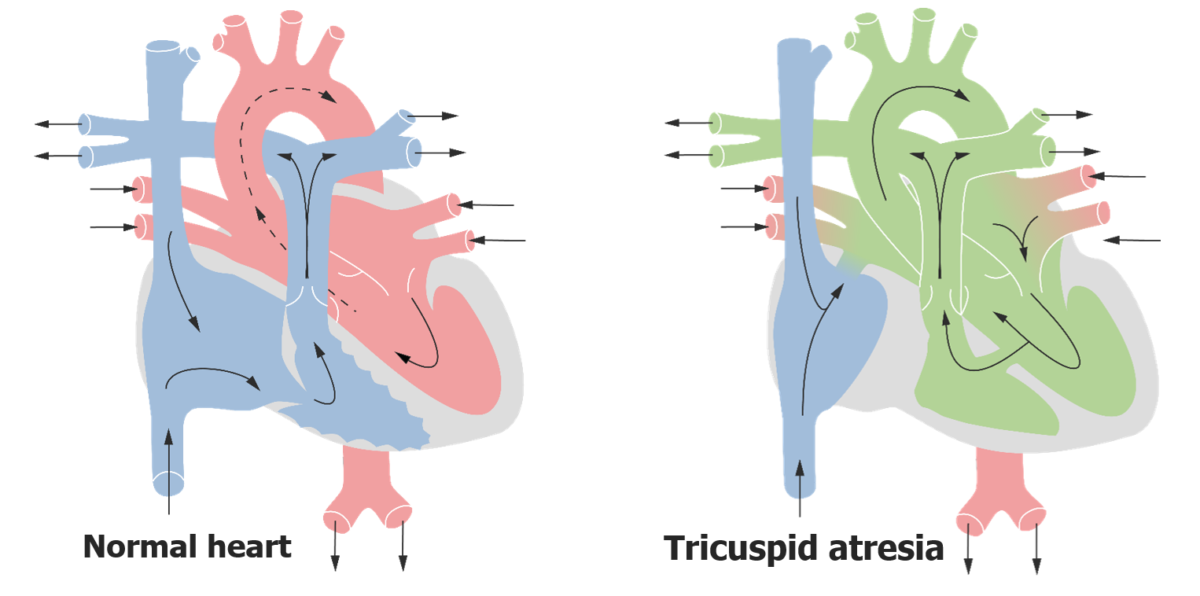
Cardiac blood flow in tricuspid atresia:
Image of blood flow through a normal heart vs. a heart with TVA. In the heart with TVA, deoxygenated blood enters the RA and flows into the LA by way of interatrial communication. The LV acts like a single chamber pumping blood into the systemic and pulmonary circulation via a VSD.
Management is medical stabilization followed by a staged surgical repair.
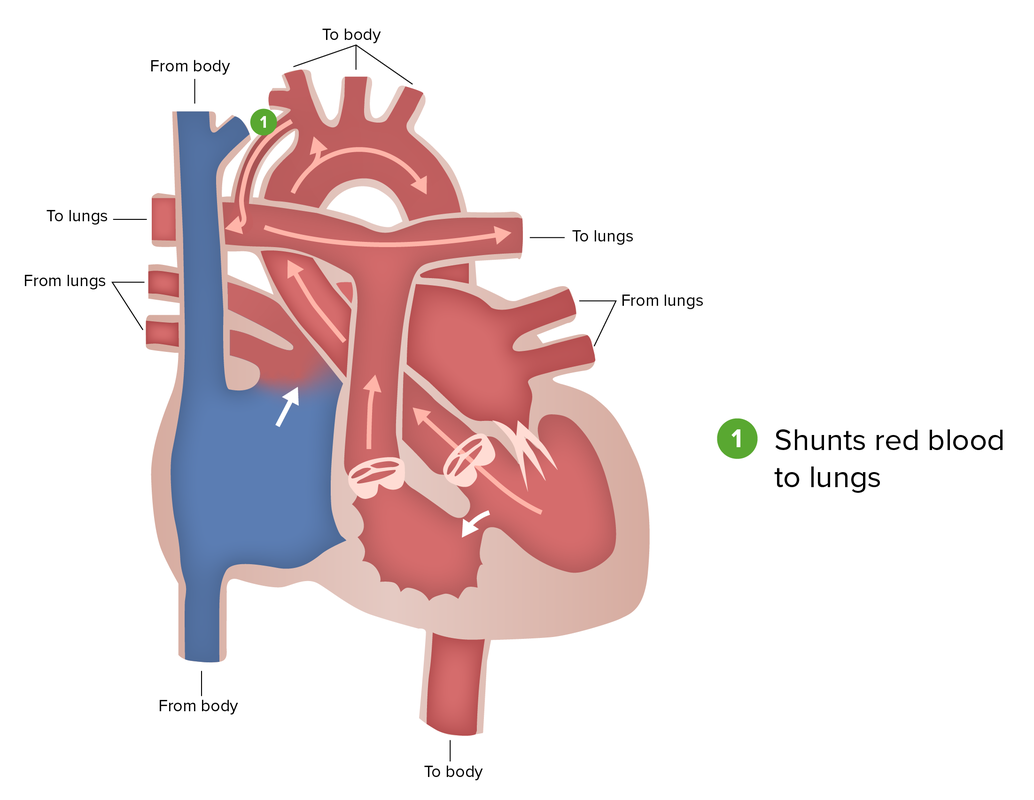
Blalock-Taussig shunt:
As the 1st in a 3-part staged surgical approach to tricuspid atresia, a Blalock-Taussig shunt seeks to mimic the function of a PDA and allow blood flow to the pulmonary circulation.
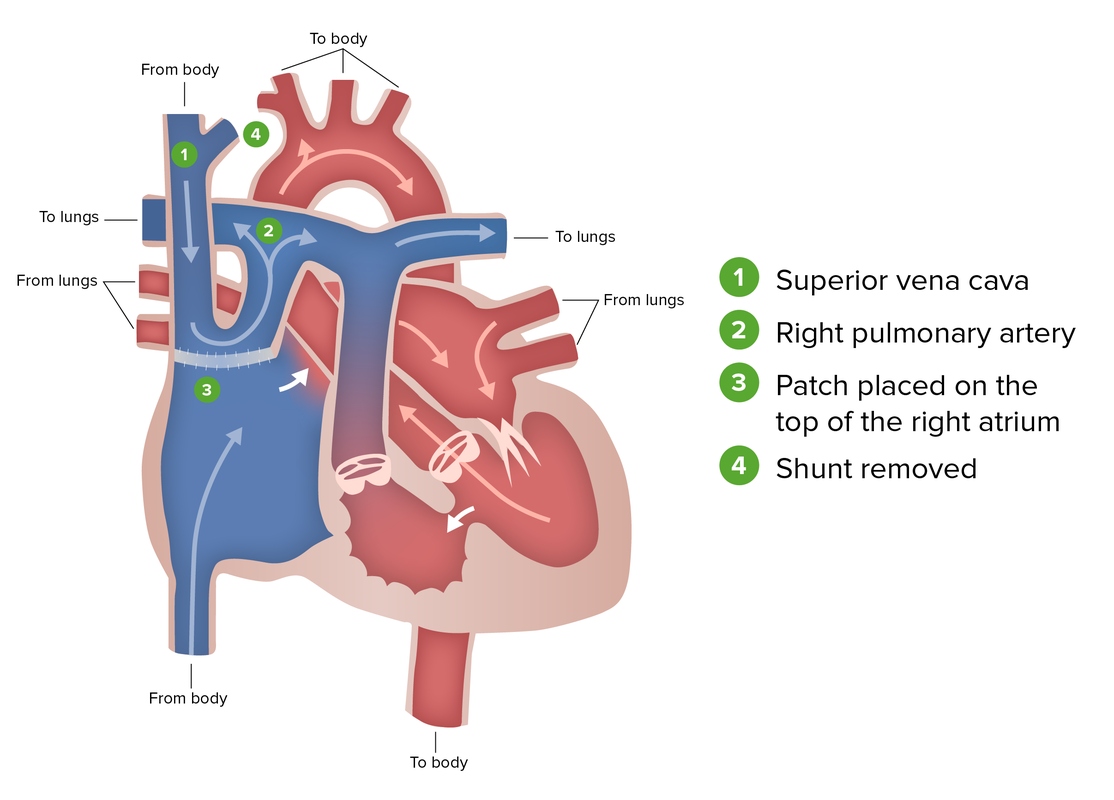
Glenn procedure (hemi Fontan):
As the 2nd in a 3-part staged surgical approach to tricuspid atresia, the Glenn procedure seeks to direct systemic venous drainage of the SVC into the PA. The procedure includes grafting the SVC into the PA and placing a patch in the RA that directs venous return from the upper body into pulmonary circulation and venous return from the lower body to the LA through a PFO. The shunt from the previous procedure is removed.
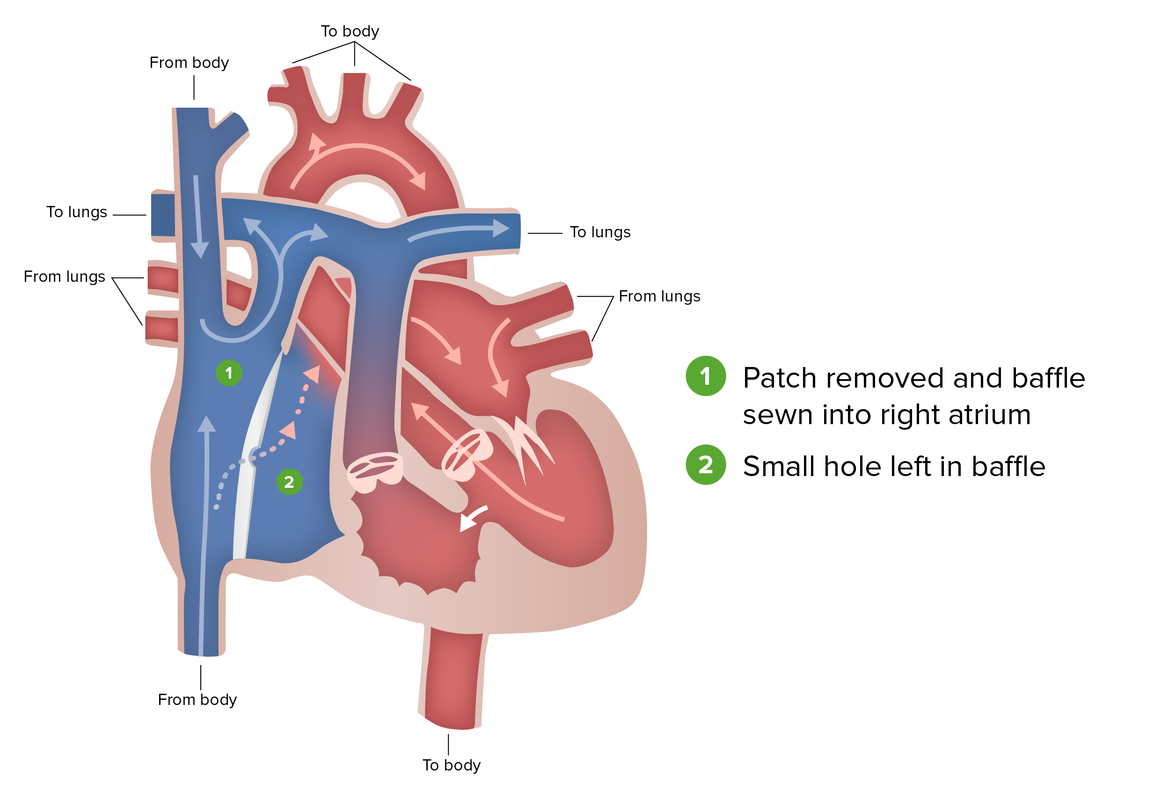
Fontan procedure:
The final in a 3-part staged surgical approach to tricuspid atresia in which the patch placed in the previous surgery is replaced with a baffle allowing venous return from both halves of the vena cava to drain into the PAs. A hole may be left to function as a pop-off valve if the pressure of the returning venous blood is too high for the pulmonary circulation.
Additional CCHD include: