Transposition of the great arteries Arteries Arteries are tubular collections of cells that transport oxygenated blood and nutrients from the heart to the tissues of the body. The blood passes through the arteries in order of decreasing luminal diameter, starting in the largest artery (the aorta) and ending in the small arterioles. Arteries are classified into 3 types: large elastic arteries, medium muscular arteries, and small arteries and arterioles. Arteries: Histology (TGA) is a cyanotic congenital heart defect Cyanotic Congenital Heart Defect Total Anomalous Pulmonary Venous Return (TAPVR) where the aorta Aorta The main trunk of the systemic arteries. Mediastinum and Great Vessels: Anatomy arises from the right ventricle and the pulmonary artery Pulmonary artery The short wide vessel arising from the conus arteriosus of the right ventricle and conveying unaerated blood to the lungs. Lungs: Anatomy from the left, creating parallel systemic and pulmonary circulations. In D-TGA (the most common variant), the aorta Aorta The main trunk of the systemic arteries. Mediastinum and Great Vessels: Anatomy is anterior/right of the pulmonary artery Pulmonary artery The short wide vessel arising from the conus arteriosus of the right ventricle and conveying unaerated blood to the lungs. Lungs: Anatomy. A rare variant, ccTGA, also exists but is far less common. Neonates present with cyanosis Cyanosis A bluish or purplish discoloration of the skin and mucous membranes due to an increase in the amount of deoxygenated hemoglobin in the blood or a structural defect in the hemoglobin molecule. Pulmonary Examination unresponsive to supplemental oxygen Supplemental Oxygen Respiratory Failure, tachypnea Tachypnea Increased respiratory rate. Pulmonary Examination, and heart failure Heart Failure A heterogeneous condition in which the heart is unable to pump out sufficient blood to meet the metabolic need of the body. Heart failure can be caused by structural defects, functional abnormalities (ventricular dysfunction), or a sudden overload beyond its capacity. Chronic heart failure is more common than acute heart failure which results from sudden insult to cardiac function, such as myocardial infarction. Total Anomalous Pulmonary Venous Return (TAPVR) (especially with a large VSD Large VSD Ventricular Septal Defect (VSD)). Diagnosis is confirmed by an echocardiogram and a chest X-ray X-ray Penetrating electromagnetic radiation emitted when the inner orbital electrons of an atom are excited and release radiant energy. X-ray wavelengths range from 1 pm to 10 nm. Hard x-rays are the higher energy, shorter wavelength x-rays. Soft x-rays or grenz rays are less energetic and longer in wavelength. The short wavelength end of the x-ray spectrum overlaps the gamma rays wavelength range. The distinction between gamma rays and x-rays is based on their radiation source. Pulmonary Function Tests showing the classic “egg on a string” pattern. Treatment is primarily surgical, and the prognosis Prognosis A prediction of the probable outcome of a disease based on a individual's condition and the usual course of the disease as seen in similar situations. Non-Hodgkin Lymphomas for surgically corrected cases is good.
Last updated: Jan 30, 2025
Transposition of the great arteries Arteries Arteries are tubular collections of cells that transport oxygenated blood and nutrients from the heart to the tissues of the body. The blood passes through the arteries in order of decreasing luminal diameter, starting in the largest artery (the aorta) and ending in the small arterioles. Arteries are classified into 3 types: large elastic arteries, medium muscular arteries, and small arteries and arterioles. Arteries: Histology (TGA) is the switching of the origins of the great vessels whereby the aorta Aorta The main trunk of the systemic arteries. Mediastinum and Great Vessels: Anatomy arises from the right ventricle and the pulmonary artery Pulmonary artery The short wide vessel arising from the conus arteriosus of the right ventricle and conveying unaerated blood to the lungs. Lungs: Anatomy arises from the left ventricle.
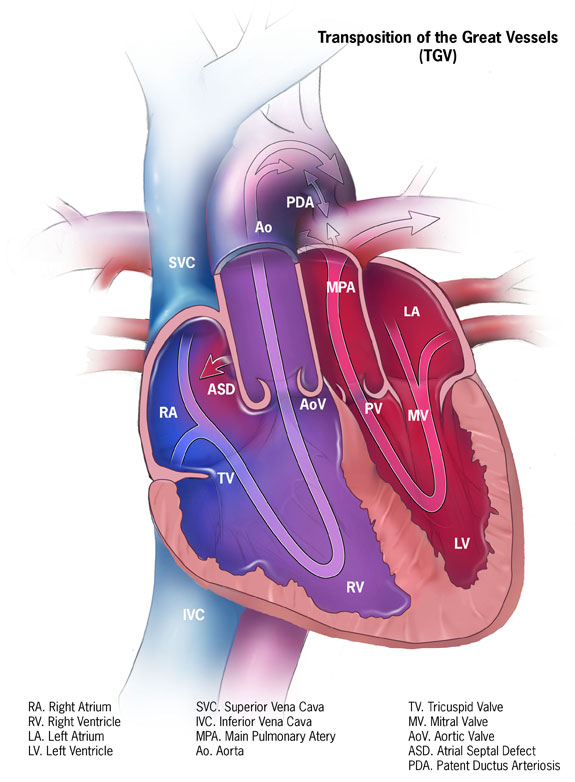
Transposition of the great arteries
Image: “Transposition of the great vessels” by Centers for Disease Control and Prevention. License: CC0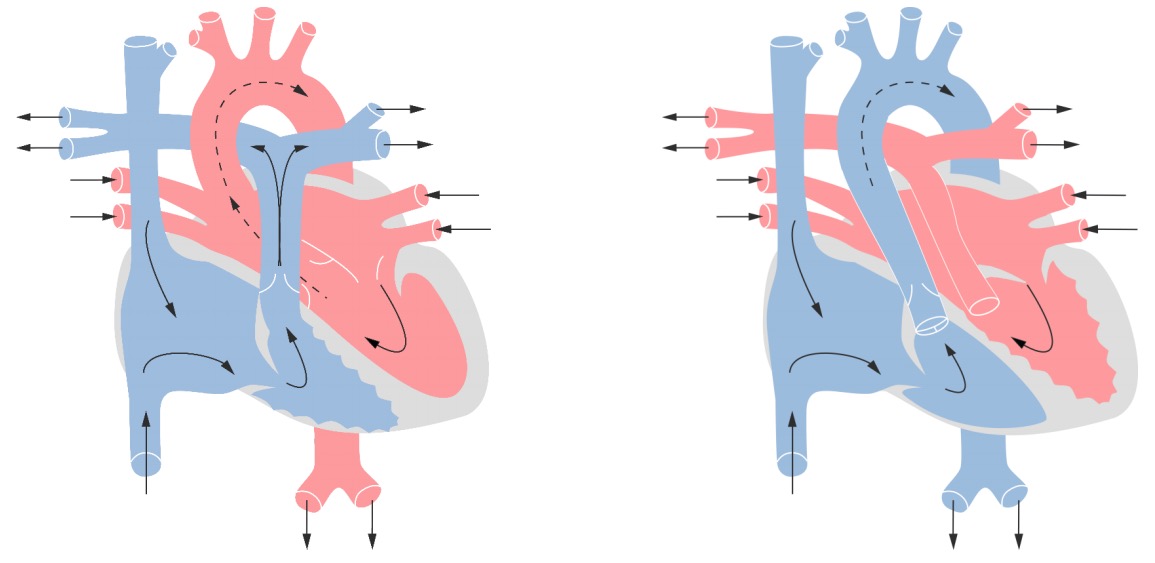
A normal heart versus a heart of a patient with TGA
The origin of the aorta is from the right ventricle carrying deoxygenated blood while the pulmonary artery originates from the left ventricle.
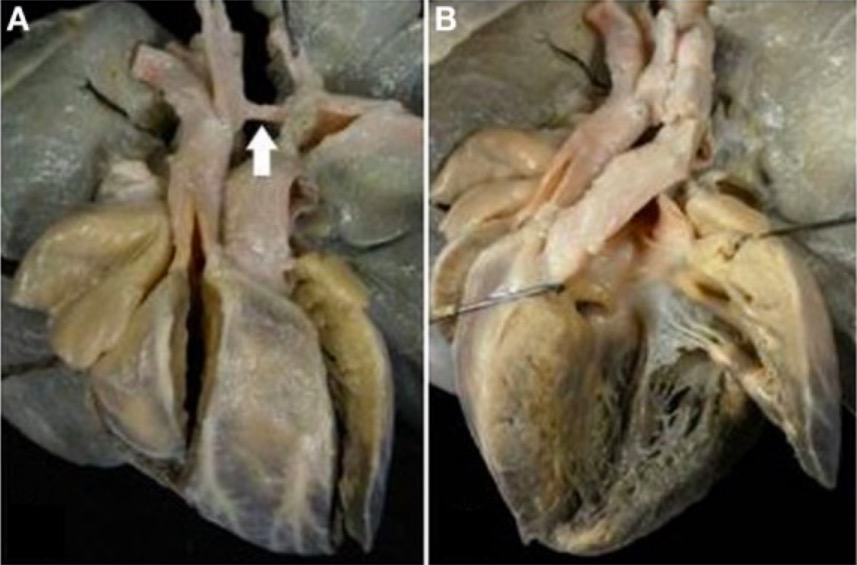
Complete TGV with VSD and aortic arch obstruction
A: External view of the heart showing the right anterior position of the aorta and the severe hypoplasia of the aortic arch between carotid and subclavian artery (arrow)
B: The pulmonary artery originates from the left ventricle. Note the malalignment of the VSD with the infundibular septum deviated to the right.
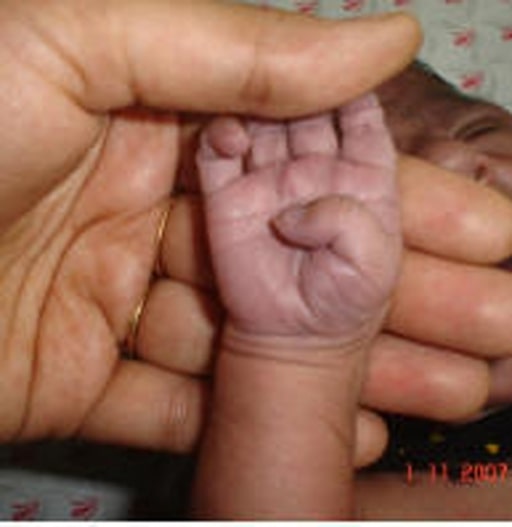
Peripheral cyanosis in a newborn
This may be the first sign of cyanosis in TGV before closure of the patent ductus arteriosus (PDA) occurs after several hours or days. A patent PDA and/or another central shunt, such as a septal defect in the atria or ventricles, may allow enough mixing to prevent severe (central) cyanosis initially, but as the PDA closes, cyanosis becomes more severe.
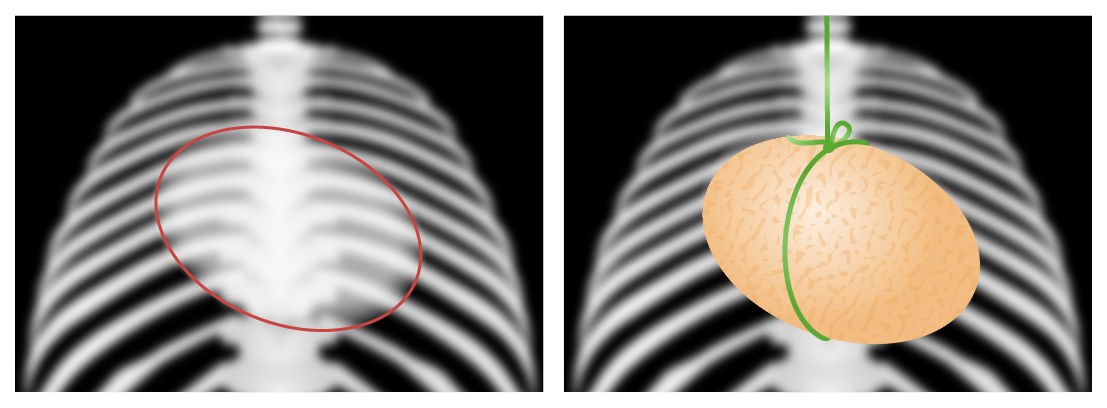
Egg-on-a-string sign
These graphic figures recreate the chest X-ray images of an infant with transposition of the great vessels, known as the “egg-on-a-string sign.” The egg on its side represents a globular-shaped heart which owes its appearance to the prominent convexity of the right atrial border and left atrial enlargement. The string represents an exaggeration of the attenuation of the superior mediastinum which is narrowed due to stress-induced thymic atrophy (and consequent loss of the normally prominent thymic shadow).
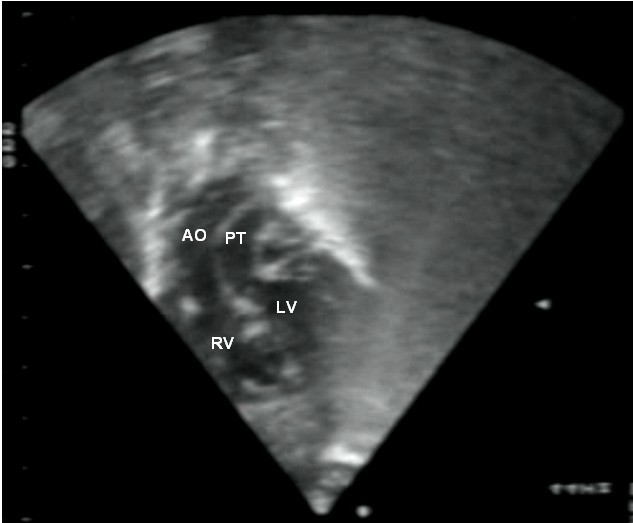
Echocardiogram showing transposition of the great arteries
This subcostal view shows discordant ventricular arterial connections together with the presence of parallel, rather than crossing, great arteries arising from the ventricles.
LV: left ventricle
RV: right ventricle
PT: pulmonary trunk
AO: aorta
Medical:
Surgery: