Ventricular septal defects (VSDs) are congenital cardiac malformations that feature an abnormal communication Communication The exchange or transmission of ideas, attitudes, or beliefs between individuals or groups. Decision-making Capacity and Legal Competence between the right and left ventricles. Presenting both in isolation or as part of a more complex disease, VSD is the most common congenital heart defect. While the degree of severity depends on the size of the defect, VSDs are classified on the basis of the anatomical location of the defect. Patients Patients Individuals participating in the health care system for the purpose of receiving therapeutic, diagnostic, or preventive procedures. Clinician–Patient Relationship may be asymptomatic with smaller defects, whereas larger defects can present with respiratory or heart failure Heart Failure A heterogeneous condition in which the heart is unable to pump out sufficient blood to meet the metabolic need of the body. Heart failure can be caused by structural defects, functional abnormalities (ventricular dysfunction), or a sudden overload beyond its capacity. Chronic heart failure is more common than acute heart failure which results from sudden insult to cardiac function, such as myocardial infarction. Total Anomalous Pulmonary Venous Return (TAPVR) during infancy or childhood. A common clinical sign is a holosystolic murmur Holosystolic Murmur Tricuspid Valve Atresia (TVA) audible at the left sternal border. Diagnosis, both pre- and post-natal, is confirmed by echocardiogram Echocardiogram Transposition of the Great Arteries. The majority of small VSDs close spontaneously, but those that are larger and symptomatic require medical stabilization followed by surgical repair.
Last updated: Feb 18, 2025
A ventricular septal defect Ventricular Septal Defect Tetralogy of Fallot (VSD) is a malformation of the interventricular septum (IVS) resulting in an abnormal communication Communication The exchange or transmission of ideas, attitudes, or beliefs between individuals or groups. Decision-making Capacity and Legal Competence between the left ventricle (LV) and the right ventricle (RV). This defect may present in isolation, or be a part of another anomaly, such as tetralogy of Fallot Tetralogy of Fallot Tetralogy of Fallot is the most common cyanotic congenital heart disease. The disease is the confluence of 4 pathologic cardiac features: overriding aorta, ventricular septal defect, right ventricular outflow obstruction, and right ventricular hypertrophy. Tetralogy of Fallot.
There are 4 main subtypes of VSD based on location along IVS:
Exact mechanism is unknown. Genetic syndromes:
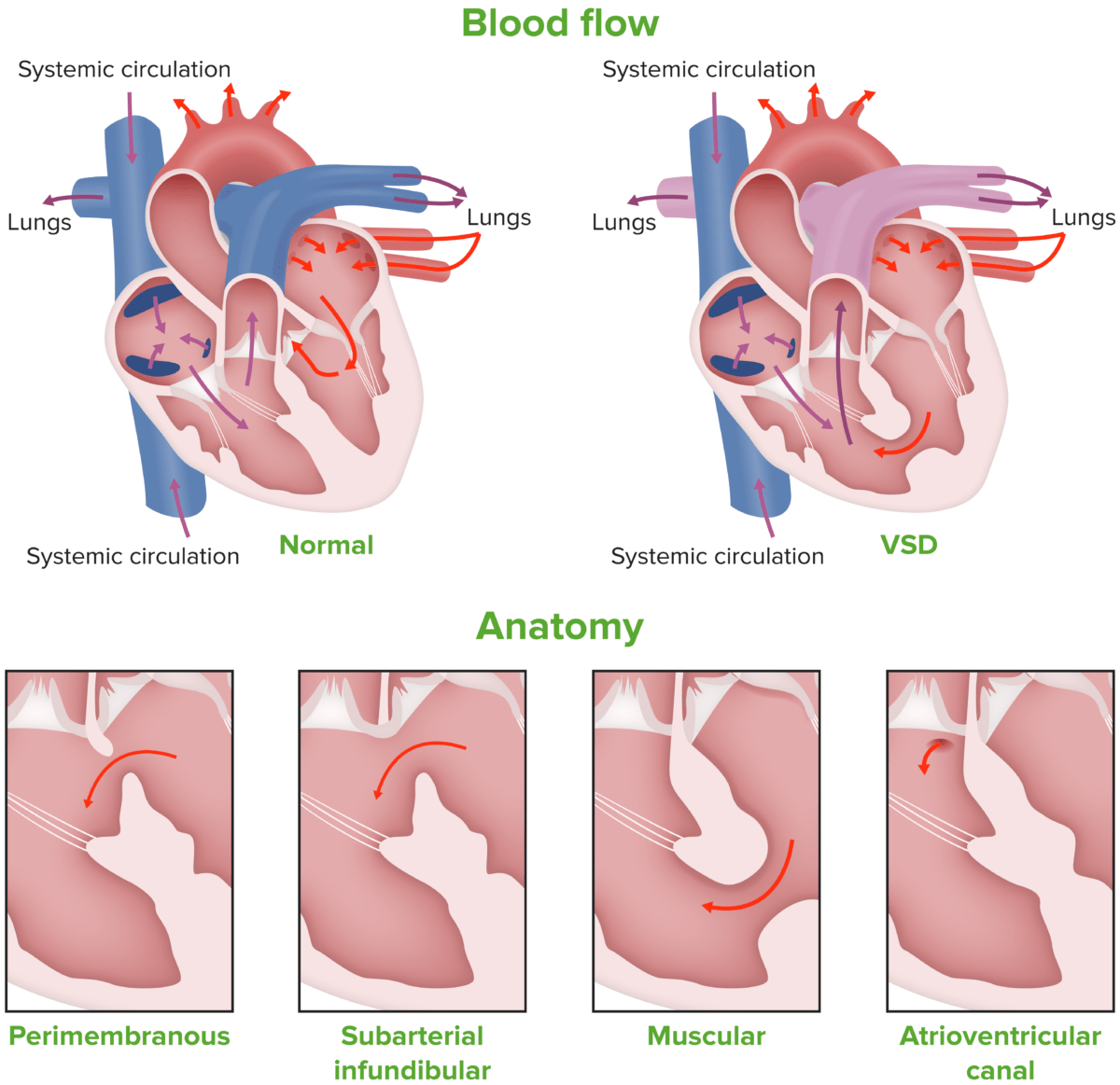
Image showing the different types of ventricular septal defect
Image by Lecturio.Physiologic changes occur based on the size of the defect and the resistance Resistance Physiologically, the opposition to flow of air caused by the forces of friction. As a part of pulmonary function testing, it is the ratio of driving pressure to the rate of air flow. Ventilation: Mechanics of Breathing across which the blood has to flow Flow Blood flows through the heart, arteries, capillaries, and veins in a closed, continuous circuit. Flow is the movement of volume per unit of time. Flow is affected by the pressure gradient and the resistance fluid encounters between 2 points. Vascular resistance is the opposition to flow, which is caused primarily by blood friction against vessel walls. Vascular Resistance, Flow, and Mean Arterial Pressure.
Age of presentation is usually around 2 months, unless a larger defect is present which becomes symptomatic within weeks of birth.
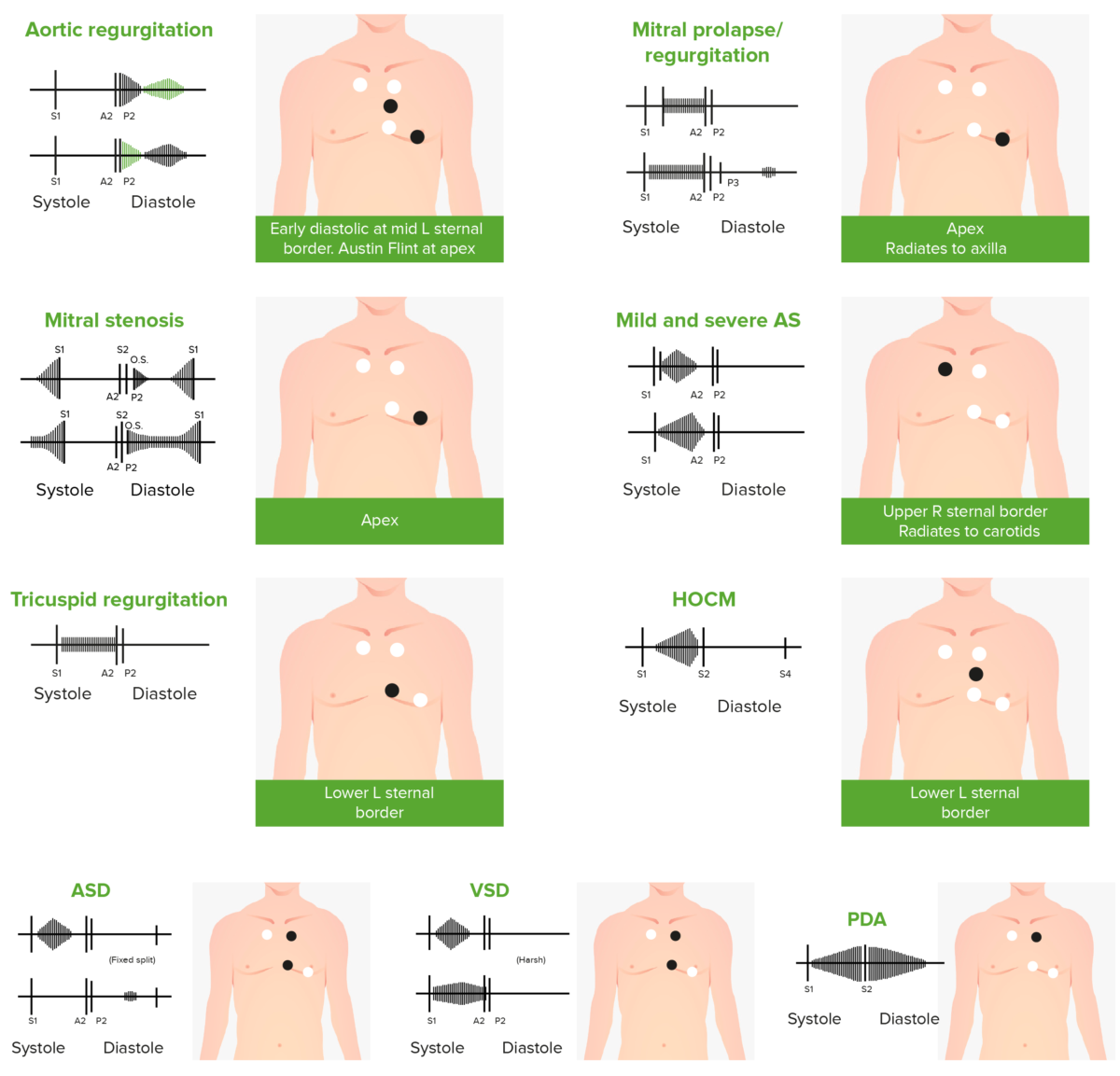
Phonocardiograms of abnormal heart sounds caused by the following cardiac defects:
Aortic regurgitation, mitral valve prolapse, mitral stenosis (MS), aortic stenosis (AS), tricuspid regurgitation, hypertrophic obstructive cardiomyopathy (HOCM), atrial septal defect (ASD), ventricular septal defect (VSD), and patent ductus arteriosus (PDA)
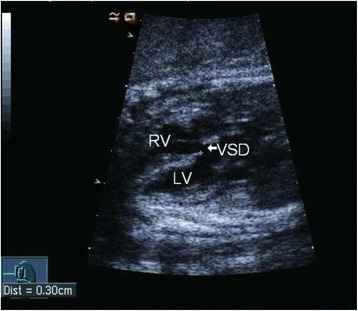
White arrow indicates a large VSD detected by fetal echocardiography.
Image: “Prediction of spontaneous closure of isolated ventricular septal defects in utero and postnatal life” by BMC Pediatrics. License: CC BY 4.0The management of a VSD is based on the size of the defect and the clinical symptoms of the patient.
The following conditions are associated with VSD and can cause or modify the disease: