Thyrotoxicosis refers to the classic physiologic manifestations of excess thyroid Thyroid The thyroid gland is one of the largest endocrine glands in the human body. The thyroid gland is a highly vascular, brownish-red gland located in the visceral compartment of the anterior region of the neck. Thyroid Gland: Anatomy hormones Hormones Hormones are messenger molecules that are synthesized in one part of the body and move through the bloodstream to exert specific regulatory effects on another part of the body. Hormones play critical roles in coordinating cellular activities throughout the body in response to the constant changes in both the internal and external environments. Hormones: Overview and Types and is not synonymous with hyperthyroidism, which is caused by sustained overproduction and release of T3 and/or T4. Graves’ disease is the most common cause of primary hyperthyroidism, followed by toxic multinodular goiter Multinodular goiter An enlarged thyroid gland containing multiple nodules (thyroid nodule), usually resulting from recurrent thyroid hyperplasia and involution over many years to produce the irregular enlargement. Multinodular goiters may be nontoxic or may induce thyrotoxicosis. Goiter and toxic adenoma. Subacute thyroiditis Thyroiditis Thyroiditis is a catchall term used to describe a variety of conditions that have inflammation of the thyroid gland in common. It includes pathologies that cause an acute illness with severe thyroid pain (e.g., subacute thyroiditis and infectious thyroiditis) as well as conditions in which there is no clinically evident inflammation and the manifestations primarily reflect thyroid dysfunction or a goiter (e.g., painless thyroiditis and fibrous Riedel's thyroiditis). Thyroiditis is an example of thyrotoxicosis without hyperthyroidism, and a pituitary Pituitary A small, unpaired gland situated in the sella turcica. It is connected to the hypothalamus by a short stalk which is called the infundibulum. Hormones: Overview and Types adenoma, which secretes thyroid-stimulating hormone Thyroid-stimulating hormone A glycoprotein hormone secreted by the adenohypophysis. Thyrotropin stimulates thyroid gland by increasing the iodide transport, synthesis and release of thyroid hormones (thyroxine and triiodothyronine). Thyroid Hormones (TSH) is an example of secondary hyperthyroidism. Clinical features of thyrotoxicosis are mostly due to an increase in the metabolic rate and overactivity of the sympathetic nervous system Nervous system The nervous system is a small and complex system that consists of an intricate network of neural cells (or neurons) and even more glial cells (for support and insulation). It is divided according to its anatomical components as well as its functional characteristics. The brain and spinal cord are referred to as the central nervous system, and the branches of nerves from these structures are referred to as the peripheral nervous system. Nervous System: Anatomy, Structure, and Classification (i.e., an increase in the β-adrenergic “tone”). Thyrotoxicosis is diagnosed by measuring the levels of TSH produced by the anterior pituitary Pituitary A small, unpaired gland situated in the sella turcica. It is connected to the hypothalamus by a short stalk which is called the infundibulum. Hormones: Overview and Types gland and unbound T4 and T3. Depending on the etiology and clinical presentation, it may be treated pharmacologically, surgically, or with radioiodine.
Last updated: May 17, 2024
Thyrotoxicosis is a condition characterized by the classic physiologic manifestations of excess thyroid Thyroid The thyroid gland is one of the largest endocrine glands in the human body. The thyroid gland is a highly vascular, brownish-red gland located in the visceral compartment of the anterior region of the neck. Thyroid Gland: Anatomy hormones Hormones Hormones are messenger molecules that are synthesized in one part of the body and move through the bloodstream to exert specific regulatory effects on another part of the body. Hormones play critical roles in coordinating cellular activities throughout the body in response to the constant changes in both the internal and external environments. Hormones: Overview and Types regardless of the cause or hormonal source. If the excessive hormones Hormones Hormones are messenger molecules that are synthesized in one part of the body and move through the bloodstream to exert specific regulatory effects on another part of the body. Hormones play critical roles in coordinating cellular activities throughout the body in response to the constant changes in both the internal and external environments. Hormones: Overview and Types are produced and released by the thyroid Thyroid The thyroid gland is one of the largest endocrine glands in the human body. The thyroid gland is a highly vascular, brownish-red gland located in the visceral compartment of the anterior region of the neck. Thyroid Gland: Anatomy gland, the condition is called hyperthyroidism.
Thyrotoxicosis due to hyperthyroidism:
Thyrotoxicosis without hyperthyroidism:
| Pathology | Condition |
|---|---|
| Thyroid-stimulating hormone Thyroid-stimulating hormone A glycoprotein hormone secreted by the adenohypophysis. Thyrotropin stimulates thyroid gland by increasing the iodide transport, synthesis and release of thyroid hormones (thyroxine and triiodothyronine). Thyroid Hormones (TSH)– receptor Receptor Receptors are proteins located either on the surface of or within a cell that can bind to signaling molecules known as ligands (e.g., hormones) and cause some type of response within the cell. Receptors antibody | Graves’ disease (diffuse hyperplasia Hyperplasia An increase in the number of cells in a tissue or organ without tumor formation. It differs from hypertrophy, which is an increase in bulk without an increase in the number of cells. Cellular Adaptation) |
| Inappropriate TSH secretion Secretion Coagulation Studies |
|
| Excess hCG secretion Secretion Coagulation Studies |
|
| Mutations in TSH receptor Receptor Receptors are proteins located either on the surface of or within a cell that can bind to signaling molecules known as ligands (e.g., hormones) and cause some type of response within the cell. Receptors or Gsα protein (G-protein alpha subunit) results in autonomous thyroid Thyroid The thyroid gland is one of the largest endocrine glands in the human body. The thyroid gland is a highly vascular, brownish-red gland located in the visceral compartment of the anterior region of the neck. Thyroid Gland: Anatomy function. |
|
| Category of causes | Pathology | Condition |
|---|---|---|
| Inflammation Inflammation Inflammation is a complex set of responses to infection and injury involving leukocytes as the principal cellular mediators in the body’s defense against pathogenic organisms. Inflammation is also seen as a response to tissue injury in the process of wound healing. The 5 cardinal signs of inflammation are pain, heat, redness, swelling, and loss of function. Inflammation and release of stored thyroid Thyroid The thyroid gland is one of the largest endocrine glands in the human body. The thyroid gland is a highly vascular, brownish-red gland located in the visceral compartment of the anterior region of the neck. Thyroid Gland: Anatomy hormone | Autoimmune destruction of thyroid Thyroid The thyroid gland is one of the largest endocrine glands in the human body. The thyroid gland is a highly vascular, brownish-red gland located in the visceral compartment of the anterior region of the neck. Thyroid Gland: Anatomy gland |
|
| Viral or postviral infection (etiology not definitive) | Subacute (painful) thyroiditis Thyroiditis Thyroiditis is a catchall term used to describe a variety of conditions that have inflammation of the thyroid gland in common. It includes pathologies that cause an acute illness with severe thyroid pain (e.g., subacute thyroiditis and infectious thyroiditis) as well as conditions in which there is no clinically evident inflammation and the manifestations primarily reflect thyroid dysfunction or a goiter (e.g., painless thyroiditis and fibrous Riedel’s thyroiditis). Thyroiditis (De Quervain thyroiditis Thyroiditis Thyroiditis is a catchall term used to describe a variety of conditions that have inflammation of the thyroid gland in common. It includes pathologies that cause an acute illness with severe thyroid pain (e.g., subacute thyroiditis and infectious thyroiditis) as well as conditions in which there is no clinically evident inflammation and the manifestations primarily reflect thyroid dysfunction or a goiter (e.g., painless thyroiditis and fibrous Riedel’s thyroiditis). Thyroiditis, granulomatous thyroiditis Thyroiditis Thyroiditis is a catchall term used to describe a variety of conditions that have inflammation of the thyroid gland in common. It includes pathologies that cause an acute illness with severe thyroid pain (e.g., subacute thyroiditis and infectious thyroiditis) as well as conditions in which there is no clinically evident inflammation and the manifestations primarily reflect thyroid dysfunction or a goiter (e.g., painless thyroiditis and fibrous Riedel’s thyroiditis). Thyroiditis) | |
| Toxic drug effects | Drug-induced thyroiditis Drug-Induced Thyroiditis Thyroiditis ( amiodarone Amiodarone An antianginal and class III antiarrhythmic drug. It increases the duration of ventricular and atrial muscle action by inhibiting potassium channels and voltage-gated sodium channels. There is a resulting decrease in heart rate and in vascular resistance. Pulmonary Fibrosis, interferon-α, checkpoint inhibitors) | |
| Bacterial or fungal infection | Acute suppurative thyroiditis Acute suppurative thyroiditis Acute inflammatory disease of the thyroid gland due to infections by bacteria; fungi; or other microorganisms. Symptoms include tender swelling, fever, and often with leukocytosis. Thyroiditis | |
| Radiation Radiation Emission or propagation of acoustic waves (sound), electromagnetic energy waves (such as light; radio waves; gamma rays; or x-rays), or a stream of subatomic particles (such as electrons; neutrons; protons; or alpha particles). Osteosarcoma | Radiation thyroiditis Radiation Thyroiditis Thyroiditis | |
| Extrathyroidal source of hormone | Excess intake of thyroid Thyroid The thyroid gland is one of the largest endocrine glands in the human body. The thyroid gland is a highly vascular, brownish-red gland located in the visceral compartment of the anterior region of the neck. Thyroid Gland: Anatomy hormone | Excess exogenous thyroid Thyroid The thyroid gland is one of the largest endocrine glands in the human body. The thyroid gland is a highly vascular, brownish-red gland located in the visceral compartment of the anterior region of the neck. Thyroid Gland: Anatomy hormone ( iatrogenic Iatrogenic Any adverse condition in a patient occurring as the result of treatment by a physician, surgeon, or other health professional, especially infections acquired by a patient during the course of treatment. Anterior Cord Syndrome or factitious) |
| Ectopic hyperthyroidism ( thyroid Thyroid The thyroid gland is one of the largest endocrine glands in the human body. The thyroid gland is a highly vascular, brownish-red gland located in the visceral compartment of the anterior region of the neck. Thyroid Gland: Anatomy hormone produced outside the thyroid Thyroid The thyroid gland is one of the largest endocrine glands in the human body. The thyroid gland is a highly vascular, brownish-red gland located in the visceral compartment of the anterior region of the neck. Thyroid Gland: Anatomy gland) | Struma ovarii (functioning thyroid Thyroid The thyroid gland is one of the largest endocrine glands in the human body. The thyroid gland is a highly vascular, brownish-red gland located in the visceral compartment of the anterior region of the neck. Thyroid Gland: Anatomy tissue in an ovarian teratoma Teratoma A true neoplasm composed of a number of different types of tissue, none of which is native to the area in which it occurs. It is composed of tissues that are derived from three germinal layers, the endoderm, mesoderm, and ectoderm. They are classified histologically as mature (benign) or immature (malignant). Imaging of the Mediastinum); functional thyroid Thyroid The thyroid gland is one of the largest endocrine glands in the human body. The thyroid gland is a highly vascular, brownish-red gland located in the visceral compartment of the anterior region of the neck. Thyroid Gland: Anatomy cancer metastases | |
| Ingestion of contaminated food | Hamburger thyrotoxicosis (meat containing thyroid Thyroid The thyroid gland is one of the largest endocrine glands in the human body. The thyroid gland is a highly vascular, brownish-red gland located in the visceral compartment of the anterior region of the neck. Thyroid Gland: Anatomy tissue) | |
| Exposure to excessive iodine Iodine A nonmetallic element of the halogen group that is represented by the atomic symbol I, atomic number 53, and atomic weight of 126. 90. It is a nutritionally essential element, especially important in thyroid hormone synthesis. In solution, it has anti-infective properties and is used topically. Thyroid Hormones | Jod–Basedow effect | Iodine-induced hyperthyroidism ( iodine Iodine A nonmetallic element of the halogen group that is represented by the atomic symbol I, atomic number 53, and atomic weight of 126. 90. It is a nutritionally essential element, especially important in thyroid hormone synthesis. In solution, it has anti-infective properties and is used topically. Thyroid Hormones, iodine-containing drugs (e.g., amiodarone Amiodarone An antianginal and class III antiarrhythmic drug. It increases the duration of ventricular and atrial muscle action by inhibiting potassium channels and voltage-gated sodium channels. There is a resulting decrease in heart rate and in vascular resistance. Pulmonary Fibrosis), radiographic contrast agents Contrast agents Substances used to allow enhanced visualization of tissues. Computed Tomography (CT)) |
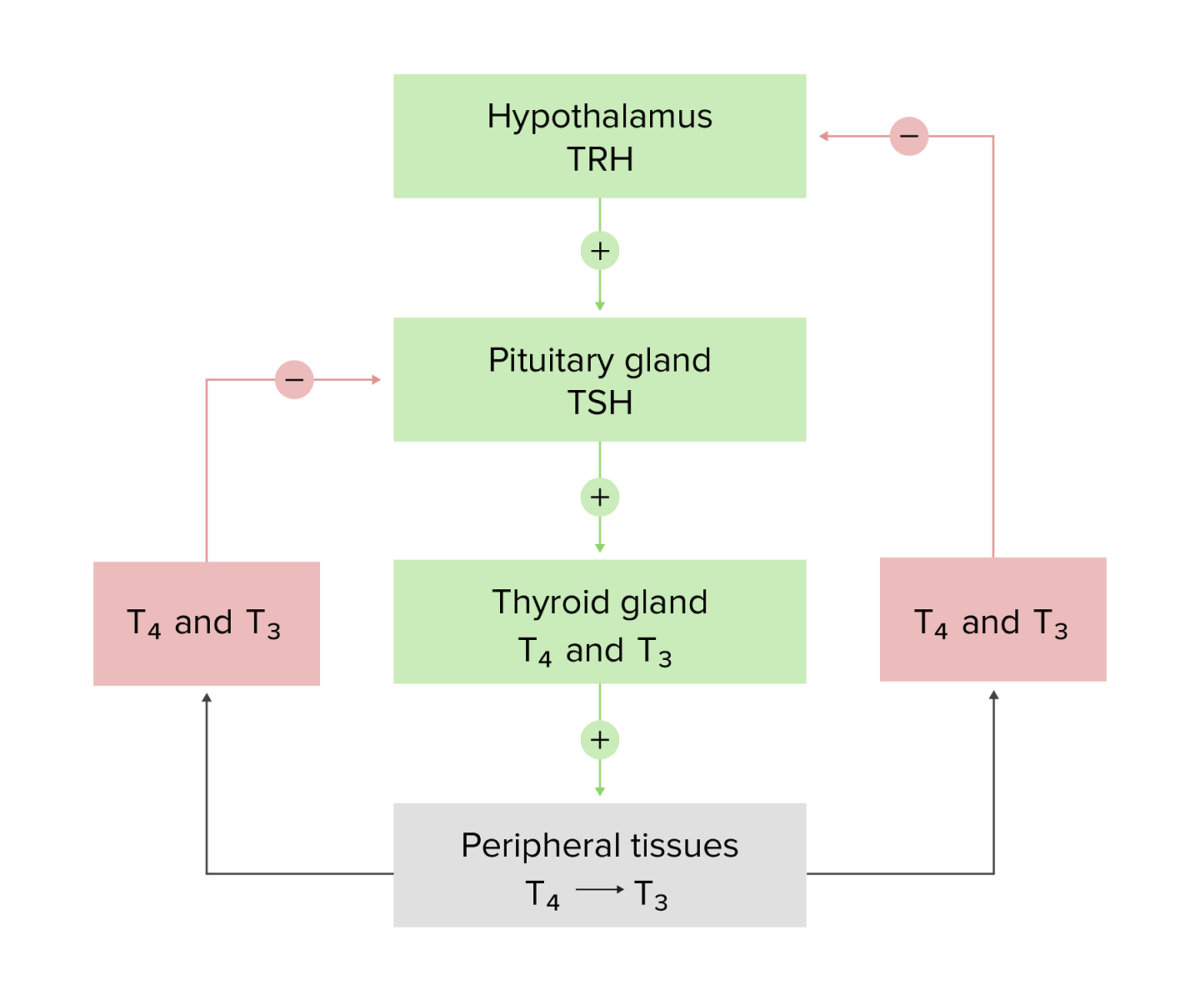
Schematic diagram of the hypothalamic–pituitary–thyroid axis and the negative feedback loops
Image by Lecturio.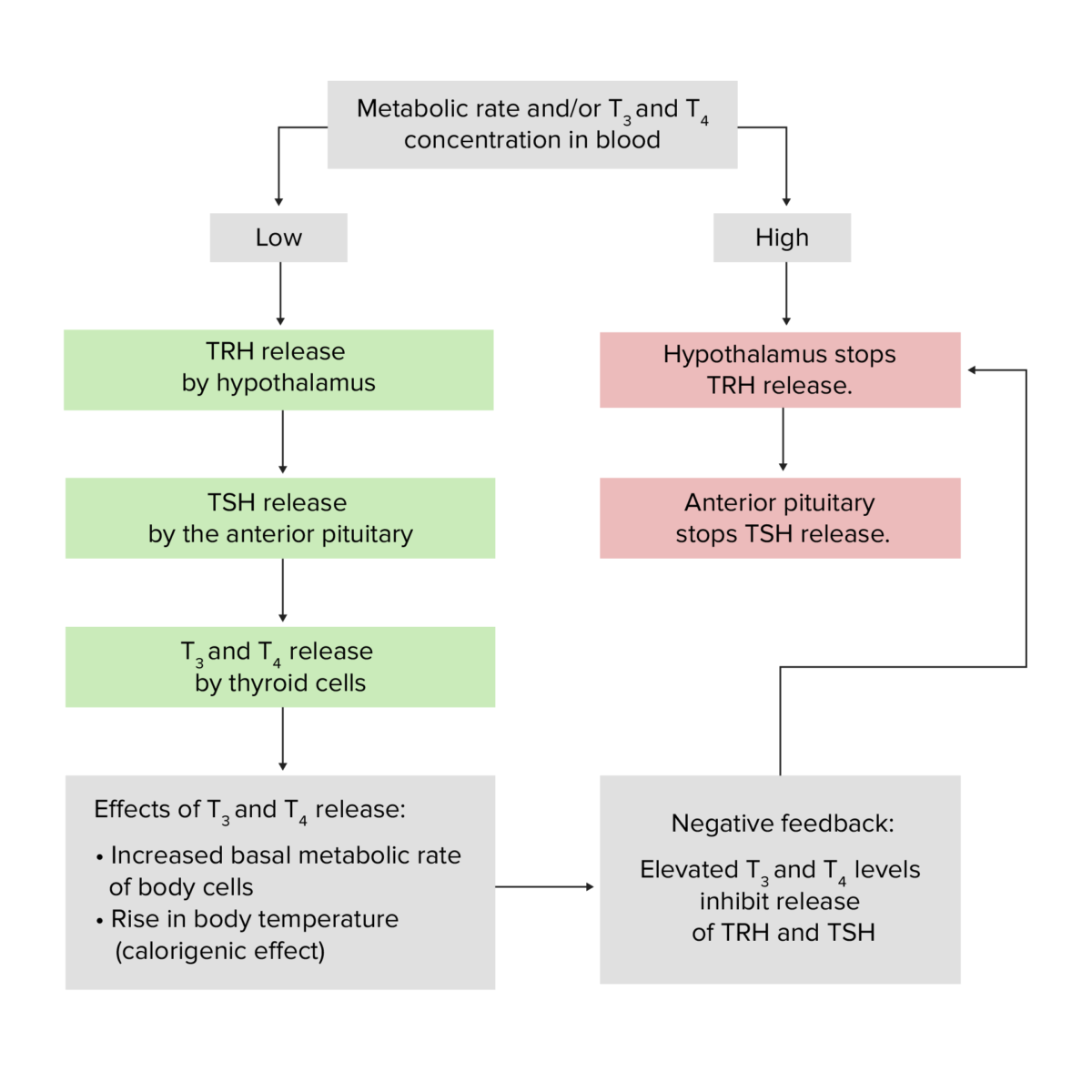
Physiology of the thyroid gland, featuring the effects of the TRH, TSH, T3, and T4 hormones as well as the basic premise of the negative hypothalamic-pituitary-thyroid gland feedback loop
Image by Lecturio.Patients Patients Individuals participating in the health care system for the purpose of receiving therapeutic, diagnostic, or preventive procedures. Clinician–Patient Relationship with high levels of T3/T4 will exhibit a compensatory decrease of TSH and a variable Variable Variables represent information about something that can change. The design of the measurement scales, or of the methods for obtaining information, will determine the data gathered and the characteristics of that data. As a result, a variable can be qualitative or quantitative, and may be further classified into subgroups. Types of Variables uptake of radioactive iodine Radioactive iodine Unstable isotopes of iodine that decay or disintegrate emitting radiation. I atoms with atomic weights 117-139, except I 127, are radioactive iodine isotopes. Antithyroid Drugs depending on the source or cause of excessive T3/T4.
With low TSH and high radioactive iodine Radioactive iodine Unstable isotopes of iodine that decay or disintegrate emitting radiation. I atoms with atomic weights 117-139, except I 127, are radioactive iodine isotopes. Antithyroid Drugs uptake (RAIU):
With low TSH and low RAIU:
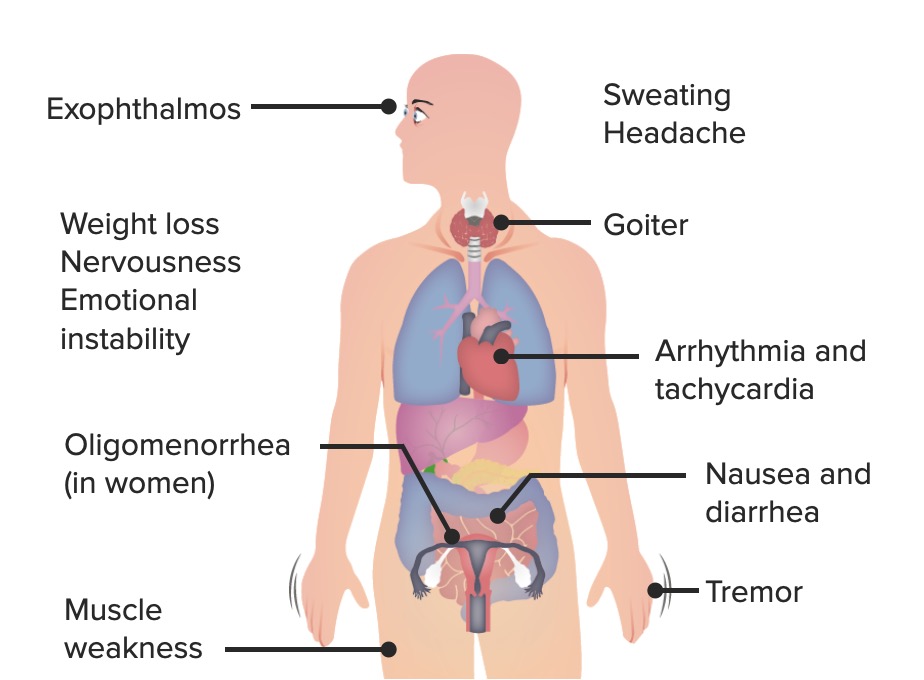
The most common signs and symptoms of hyperthyroidism: Exophthalmos is a
specific sign of Graves disease.
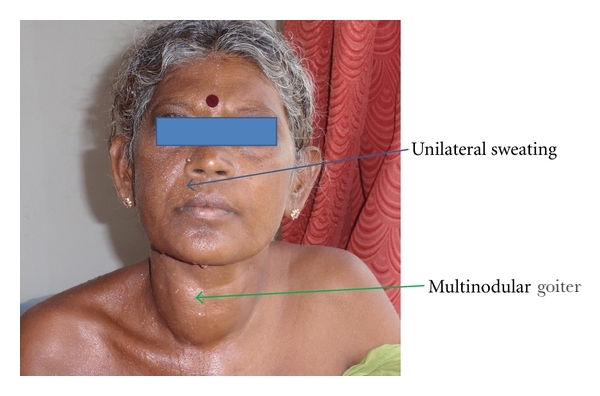
Multinodular goiter and symptoms of hyperthyroidism:
This is a 36-year-old woman who presented with a multinodular goiter and symptoms of hyperthyroidism for five years, with tachycardia and tremors in the extremities.
Increased sweating, a common sign in hyperthyroidism, was only present on the right side of the face in this rare case because the left sympathetic nerve trunk, which innervates the sweat glands and blood vessels of the left side of the face, was compressed and compromised by a dilated and tortuous left inferior thyroid artery. This is called “Harlequin syndrome” and is associated with multiple conditions. In this case it was due to an indirect consequence of the increased vascularity which accompanies an enlarged and metabolically active thyroid gland.
Cardiovascular system alterations are caused by increased circulatory demand generated by hypermetabolism/ heat production Heat Production Fever:
Gastrointestinal alterations manifest in hepatic dysfunction:
Orbitopathy and dermopathy:
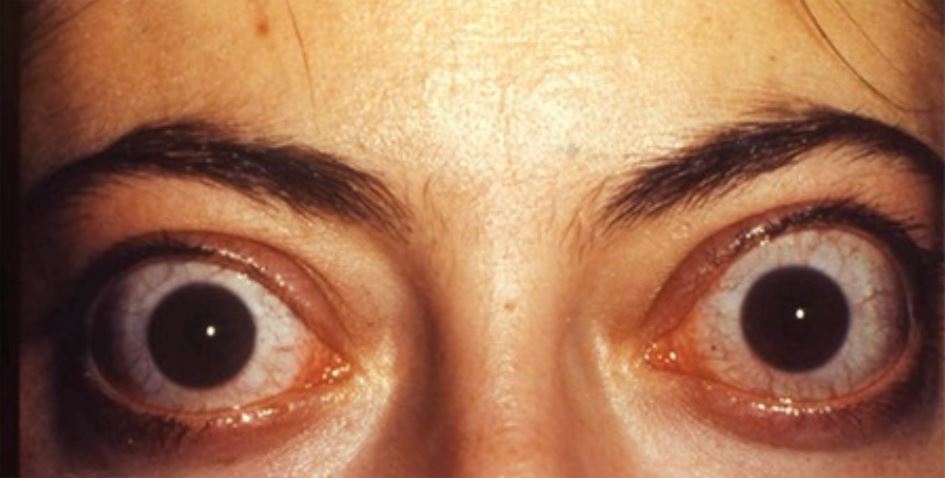
Bilateral infiltrative thyroid-associated ophthalmopathy in a 33-year-old woman
Image: “Thyroid-associated Ophthalmopathy” by Turkish Journal of Ophthalmology. License: CC BY 2.5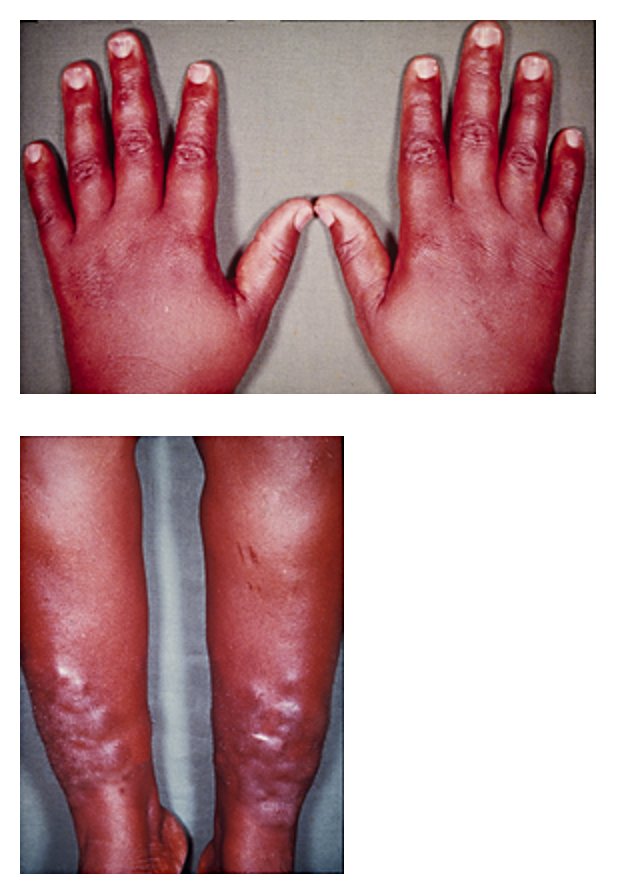
Pretibial myxedema and thyroid acropachy accompanying hyperthyroidism:
This 33-year-old woman presented with painless swelling of her fingers and lower legs of about 4 months’ duration.
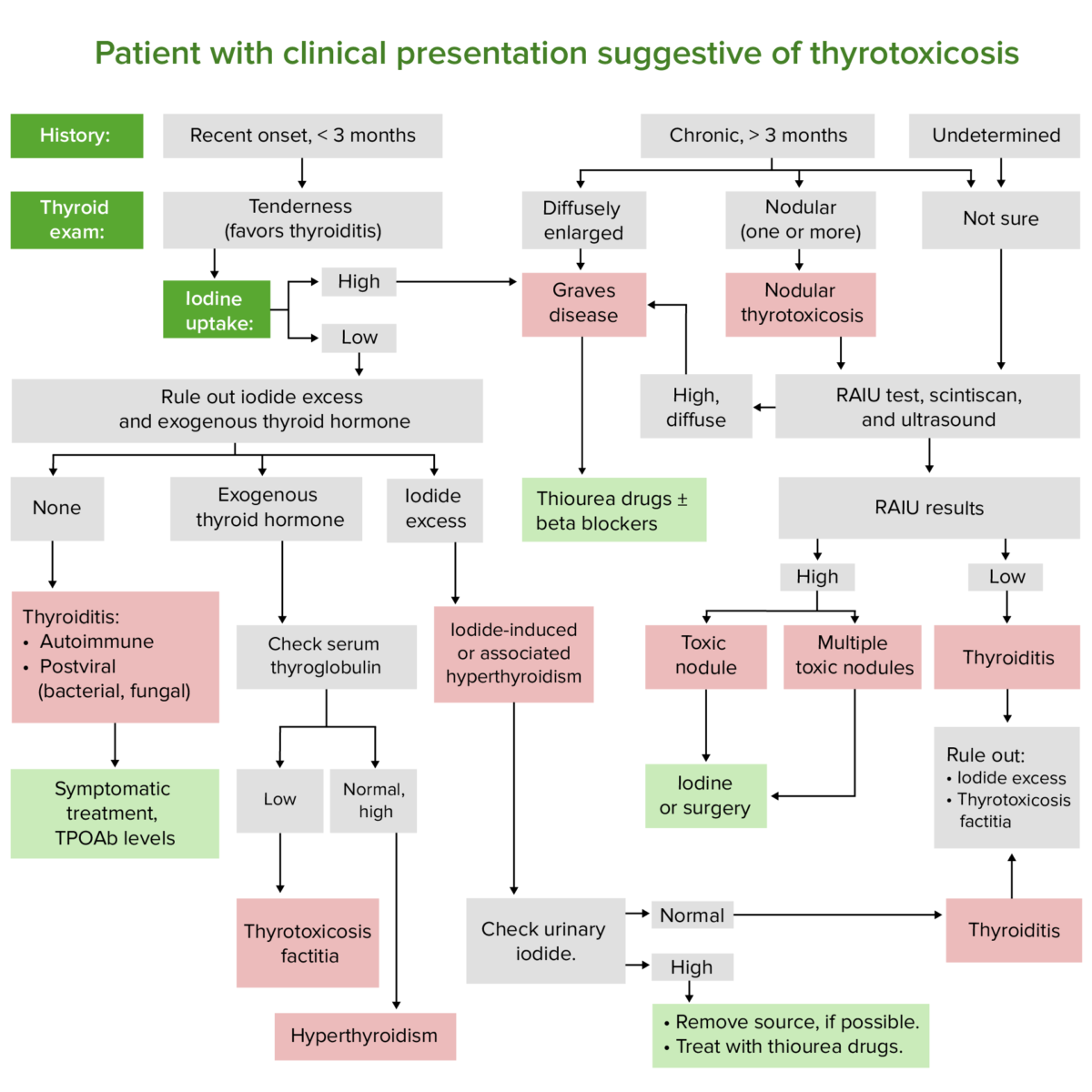
Diagnostic algorithm for patients with symptoms suggesting thyrotoxicosis
Image by Lecturio.Measurement of TSH levels is the best test for thyroid Thyroid The thyroid gland is one of the largest endocrine glands in the human body. The thyroid gland is a highly vascular, brownish-red gland located in the visceral compartment of the anterior region of the neck. Thyroid Gland: Anatomy disease screening Screening Preoperative Care and function assessment.
Thyrotoxicosis caused by autoimmune diseases Autoimmune diseases Disorders that are characterized by the production of antibodies that react with host tissues or immune effector cells that are autoreactive to endogenous peptides. Selective IgA Deficiency requires additional tests:
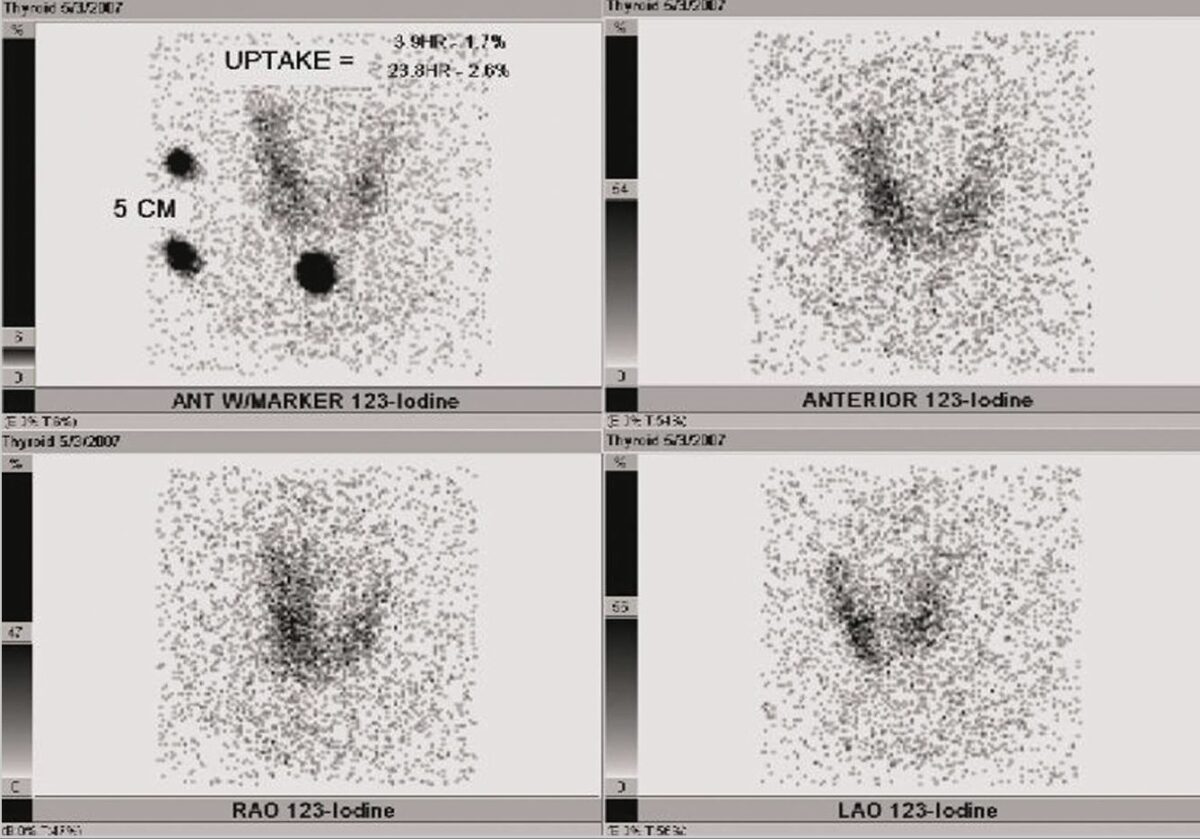
Radioactive iodine uptake (RAIU) of the thyroid of a patient with transient thyrotoxicosis:
The patient’s RAIU shows a reduced 24-hour uptake and little uptake throughout the thyroid gland.
Thyroidectomy Thyroidectomy Surgical removal of the thyroid gland. Goiter is indicated when medical treatment fails or is contraindicated owing to side effects, pregnancy Pregnancy The status during which female mammals carry their developing young (embryos or fetuses) in utero before birth, beginning from fertilization to birth. Pregnancy: Diagnosis, Physiology, and Care, or age or in the case of malignancy Malignancy Hemothorax.
Thyroid Thyroid The thyroid gland is one of the largest endocrine glands in the human body. The thyroid gland is a highly vascular, brownish-red gland located in the visceral compartment of the anterior region of the neck. Thyroid Gland: Anatomy storm is a rare, acute complication of thyrotoxicosis that constitutes a life-threatening emergency with a high mortality Mortality All deaths reported in a given population. Measures of Health Status rate.