The renal system is responsible for eliminating the daily load of non-volatile acids Acids Chemical compounds which yield hydrogen ions or protons when dissolved in water, whose hydrogen can be replaced by metals or basic radicals, or which react with bases to form salts and water (neutralization). An extension of the term includes substances dissolved in media other than water. Acid-Base Balance, which is approximately 70 millimoles per day. This daily load comes primarily from anaerobic metabolism, absorption Absorption Absorption involves the uptake of nutrient molecules and their transfer from the lumen of the GI tract across the enterocytes and into the interstitial space, where they can be taken up in the venous or lymphatic circulation. Digestion and Absorption of acids Acids Chemical compounds which yield hydrogen ions or protons when dissolved in water, whose hydrogen can be replaced by metals or basic radicals, or which react with bases to form salts and water (neutralization). An extension of the term includes substances dissolved in media other than water. Acid-Base Balance, and excretion of bases Bases Usually a hydroxide of lithium, sodium, potassium, rubidium or cesium, but also the carbonates of these metals, ammonia, and the amines. Acid-Base Balance from the GI system. Metabolic acidosis Acidosis A pathologic condition of acid accumulation or depletion of base in the body. The two main types are respiratory acidosis and metabolic acidosis, due to metabolic acid build up. Respiratory Acidosis occurs when there is an increase in the levels of new non-volatile acids Acids Chemical compounds which yield hydrogen ions or protons when dissolved in water, whose hydrogen can be replaced by metals or basic radicals, or which react with bases to form salts and water (neutralization). An extension of the term includes substances dissolved in media other than water. Acid-Base Balance (e.g., lactic acid), renal loss of HCO3-, or ingestion of toxic alcohols. Respiratory compensation Respiratory compensation Metabolic Alkalosis occurs very quickly (within minutes) and mitigates changes in pH pH The quantitative measurement of the acidity or basicity of a solution. Acid-Base Balance. In the acute period, metabolic disorders can cause severe symptoms. Management is aimed at correcting the underlying etiology.
Last updated: Mar 29, 2023
Metabolic acidosis Acidosis A pathologic condition of acid accumulation or depletion of base in the body. The two main types are respiratory acidosis and metabolic acidosis, due to metabolic acid build up. Respiratory Acidosis is the process that results in the gain of hydrogen ions (H+) or the loss of HCO3–. In primary metabolic acidosis Acidosis A pathologic condition of acid accumulation or depletion of base in the body. The two main types are respiratory acidosis and metabolic acidosis, due to metabolic acid build up. Respiratory Acidosis, arterial blood gas Arterial blood gas Respiratory Alkalosis will show:
Acid-base disorders are classified according to the primary disturbance (respiratory or metabolic) and the presence or absence of compensation Compensation Respiratory Acidosis.
Consider pH pH The quantitative measurement of the acidity or basicity of a solution. Acid-Base Balance, PCO2, and HCO3– to determine the primary disturbance.
When a patient develops acidosis Acidosis A pathologic condition of acid accumulation or depletion of base in the body. The two main types are respiratory acidosis and metabolic acidosis, due to metabolic acid build up. Respiratory Acidosis or alkalosis Alkalosis A pathological condition that removes acid or adds base to the body fluids. Respiratory Alkalosis, the body will try to compensate. Oftentimes, compensation Compensation Respiratory Acidosis will result in normal pH pH The quantitative measurement of the acidity or basicity of a solution. Acid-Base Balance.
Metabolic acidosis Acidosis A pathologic condition of acid accumulation or depletion of base in the body. The two main types are respiratory acidosis and metabolic acidosis, due to metabolic acid build up. Respiratory Acidosis is caused by 1 of 3 primary processes, namely, an increase in acid production, a loss of base, or impaired acid excretion.
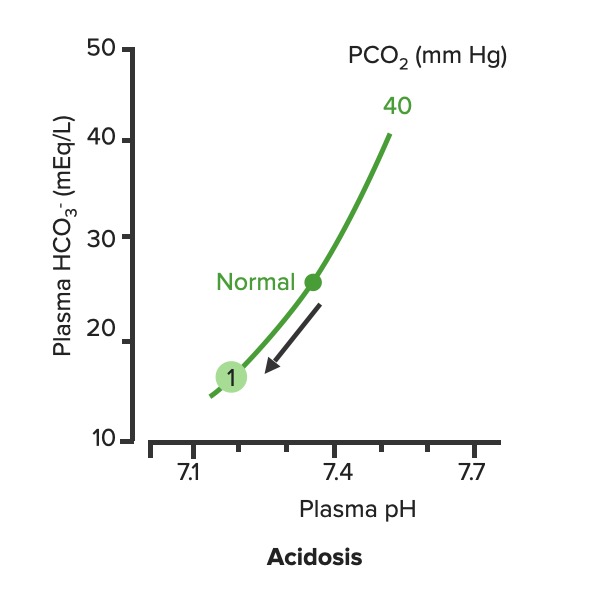
Relationship between plasma pH and plasma HCO3– in uncompensated metabolic acidosis (1):
Notice how the decrease in HCO3– moves along the PCO2.
Compensatory respiratory alkalosis Alkalosis A pathological condition that removes acid or adds base to the body fluids. Respiratory Alkalosis occurs in response to metabolic acidosis Acidosis A pathologic condition of acid accumulation or depletion of base in the body. The two main types are respiratory acidosis and metabolic acidosis, due to metabolic acid build up. Respiratory Acidosis.
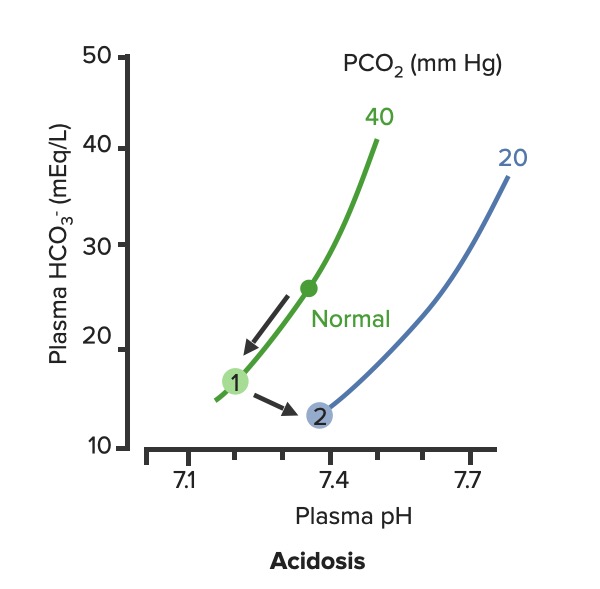
Respiratory compensation of metabolic acidosis:
As PCO2 decreases, the curve shifts down and to the right along the “blood-buffer line” (2). As the curve shifts, the pH increases toward normal.
The clinical presentation depends on the underlying etiology. Symptoms may include:
Metabolic acidosis Acidosis A pathologic condition of acid accumulation or depletion of base in the body. The two main types are respiratory acidosis and metabolic acidosis, due to metabolic acid build up. Respiratory Acidosis has a long list of potential etiologies, many of which can be present concurrently. The concepts of anion gap (AG) and osmolal gap are helpful when the diagnosis is not evident from history alone.
The AG can be used to narrow the differential diagnosis.
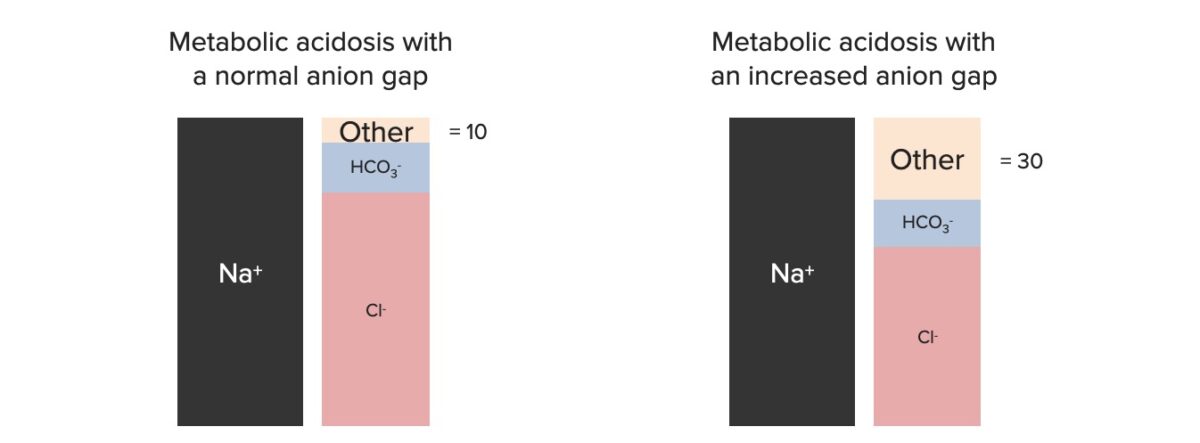
Components of the anion gap
Image by Lecturio.| Increased anion gap |
|
|---|---|
| Normal anion gap (hyperchloremic) |
|