Anticonvulsant Anticonvulsant Anticonvulsant drugs are pharmacological agents used to achieve seizure control and/or prevent seizure episodes. Anticonvulsants encompass various drugs with different mechanisms of action including ion-channel (Na+ and Ca+2) blocking and GABA reuptake inhibition. First-Generation Anticonvulsant Drugs drugs are pharmacological agents used for seizure control and/or to prevent seizure episodes. Anticonvulsants encompass various drugs with different mechanisms of action, including ion-channel (Na+, calcium Calcium A basic element found in nearly all tissues. It is a member of the alkaline earth family of metals with the atomic symbol ca, atomic number 20, and atomic weight 40. Calcium is the most abundant mineral in the body and combines with phosphorus to form calcium phosphate in the bones and teeth. It is essential for the normal functioning of nerves and muscles and plays a role in blood coagulation (as factor IV) and in many enzymatic processes. Electrolytes) blocking and inhibition of GABA GABA The most common inhibitory neurotransmitter in the central nervous system. Receptors and Neurotransmitters of the CNS reuptake. Second-generation anticonvulsants exert their effects via these mechanisms and are associated with good efficacy, fewer toxic effects, and better tolerability, and generally do not require blood level monitoring. Medications in this class include felbamate, gabapentin, pregabalin, lamotrigine, levetiracetam, oxcarbazepine Oxcarbazepine A carbamazepine derivative that acts as a voltage-gated sodium channel blocker. It is used for the treatment of partial seizures with or without secondary generalization. It is also an inducer of cytochrome p-450 cyp3a4. First-Generation Anticonvulsant Drugs, tiagabine, topiramate, zonisamide, and lacosamide. Anticonvulsant Anticonvulsant Anticonvulsant drugs are pharmacological agents used to achieve seizure control and/or prevent seizure episodes. Anticonvulsants encompass various drugs with different mechanisms of action including ion-channel (Na+ and Ca+2) blocking and GABA reuptake inhibition. First-Generation Anticonvulsant Drugs drugs are indicated for focal seizures Focal Seizures Seizures in Children, generalized tonic-clonic seizures Generalized tonic-clonic seizures A generalized seizure disorder characterized by recurrent major motor seizures. The initial brief tonic phase is marked by trunk flexion followed by diffuse extension of the trunk and extremities. The clonic phase features rhythmic flexor contractions of the trunk and limbs, pupillary dilation, elevations of blood pressure and pulse, urinary incontinence, and tongue biting. This is followed by a profound state of depressed consciousness (post-ictal state) which gradually improves over minutes to hours. The disorder may be cryptogenic, familial, or symptomatic (caused by an identified disease process). Epilepsy, myoclonic seizures Myoclonic Seizures Seizures in Children, and Lennox-Gastaut syndrome Lennox-Gastaut Syndrome Epilepsy. Some anticonvulsants are also indicated in conditions unrelated to seizures Seizures A seizure is abnormal electrical activity of the neurons in the cerebral cortex that can manifest in numerous ways depending on the region of the brain affected. Seizures consist of a sudden imbalance that occurs between the excitatory and inhibitory signals in cortical neurons, creating a net excitation. The 2 major classes of seizures are focal and generalized. Seizures (e.g., bipolar Bipolar Nervous System: Histology disorder). The most common adverse effects include dizziness Dizziness An imprecise term which may refer to a sense of spatial disorientation, motion of the environment, or lightheadedness. Lateral Medullary Syndrome (Wallenberg Syndrome), headache Headache The symptom of pain in the cranial region. It may be an isolated benign occurrence or manifestation of a wide variety of headache disorders. Brain Abscess, and somnolence.
Last updated: Mar 24, 2025
Antiseizure drugs (ASDs) are used to suppress abnormal electrical activity in the brain Brain The part of central nervous system that is contained within the skull (cranium). Arising from the neural tube, the embryonic brain is comprised of three major parts including prosencephalon (the forebrain); mesencephalon (the midbrain); and rhombencephalon (the hindbrain). The developed brain consists of cerebrum; cerebellum; and other structures in the brain stem. Nervous System: Anatomy, Structure, and Classification through various mechanisms.
The hyperexcitable state of neurons Neurons The basic cellular units of nervous tissue. Each neuron consists of a body, an axon, and dendrites. Their purpose is to receive, conduct, and transmit impulses in the nervous system. Nervous System: Histology results from 3 steps:
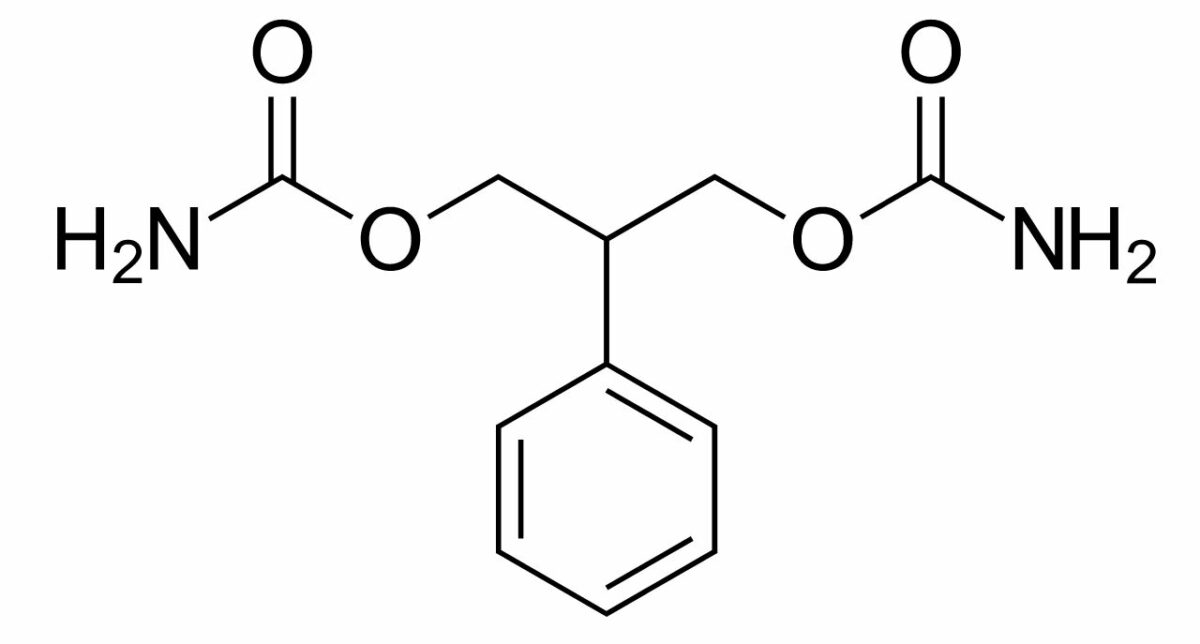
Chemical structure of felbamate
Image: “Felbamate” by Harbin. License: Public Domain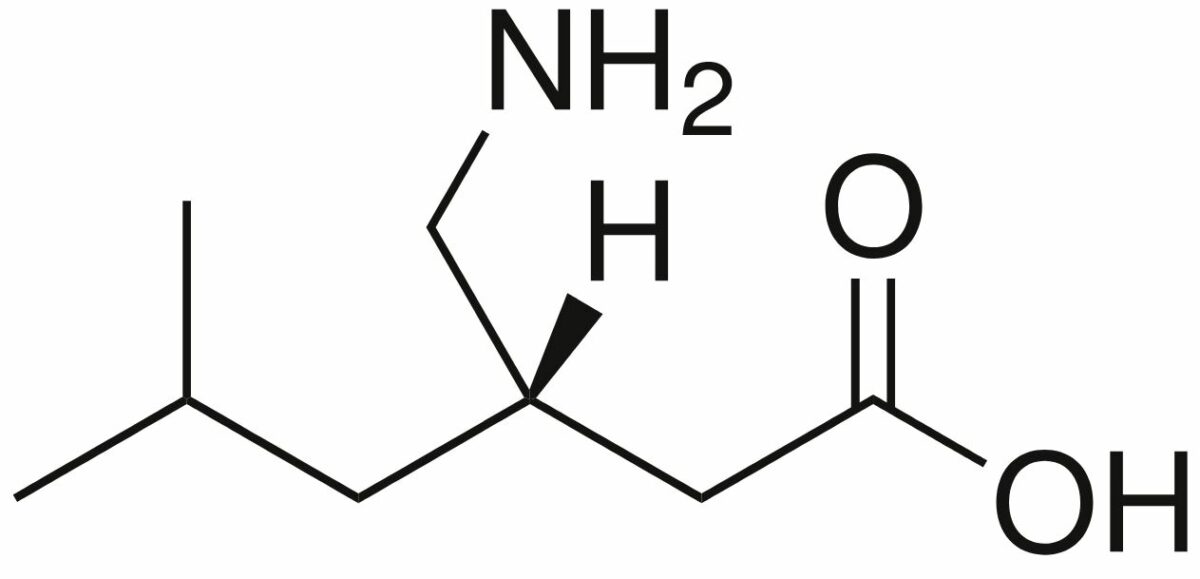
Chemical structure of pregabalin
Image: “Pregabalin” by Harbin. License: Public Domain| Drug | Pharmacokinetics Pharmacokinetics Pharmacokinetics is the science that analyzes how the human body interacts with a drug. Pharmacokinetics examines how the drug is absorbed, distributed, metabolized, and excreted by the body. Pharmacokinetics and Pharmacodynamics | Indications |
|---|---|---|
| Gabapentin |
|
|
| Pregabalin |
|
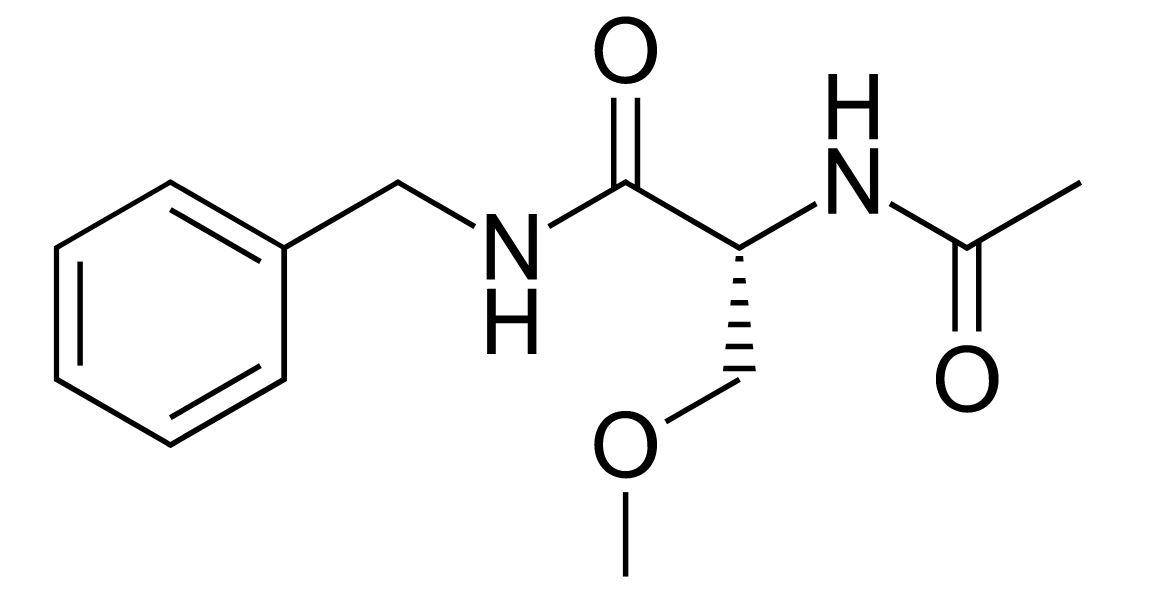
Chemical structure of lacosamide
Image: “Lacosamide” by Fvasconcellos. License: Public Domain
Chemical structure of lamotrigine
Image: “Lamotrigine” by Harbin. License: Public Domain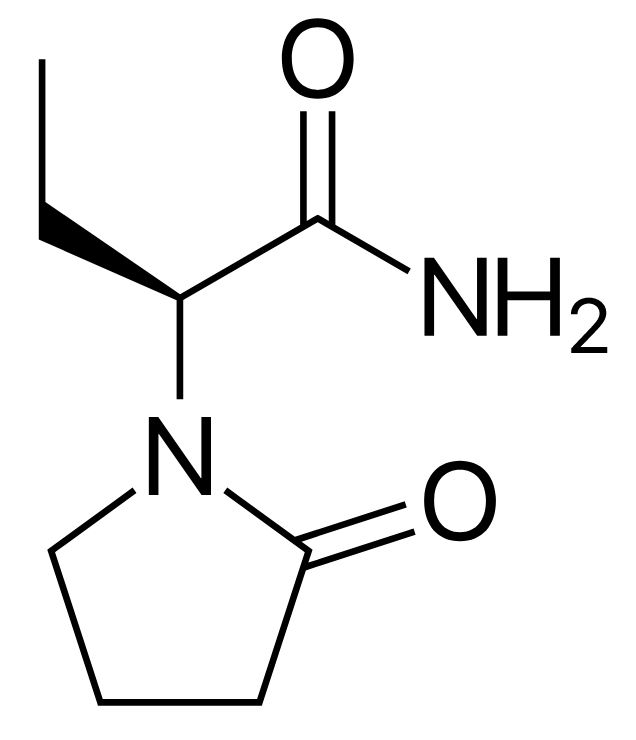
Chemical structure of levetiracetam
Image: “Levetiracetam” by Harbin. License: Public Domain| Drug | Pharmacokinetics Pharmacokinetics Pharmacokinetics is the science that analyzes how the human body interacts with a drug. Pharmacokinetics examines how the drug is absorbed, distributed, metabolized, and excreted by the body. Pharmacokinetics and Pharmacodynamics | Indications |
|---|---|---|
| Levetiracetam |
|
|
| Brivaracetam |
|
Focal (partial) seizures Seizures A seizure is abnormal electrical activity of the neurons in the cerebral cortex that can manifest in numerous ways depending on the region of the brain affected. Seizures consist of a sudden imbalance that occurs between the excitatory and inhibitory signals in cortical neurons, creating a net excitation. The 2 major classes of seizures are focal and generalized. Seizures |
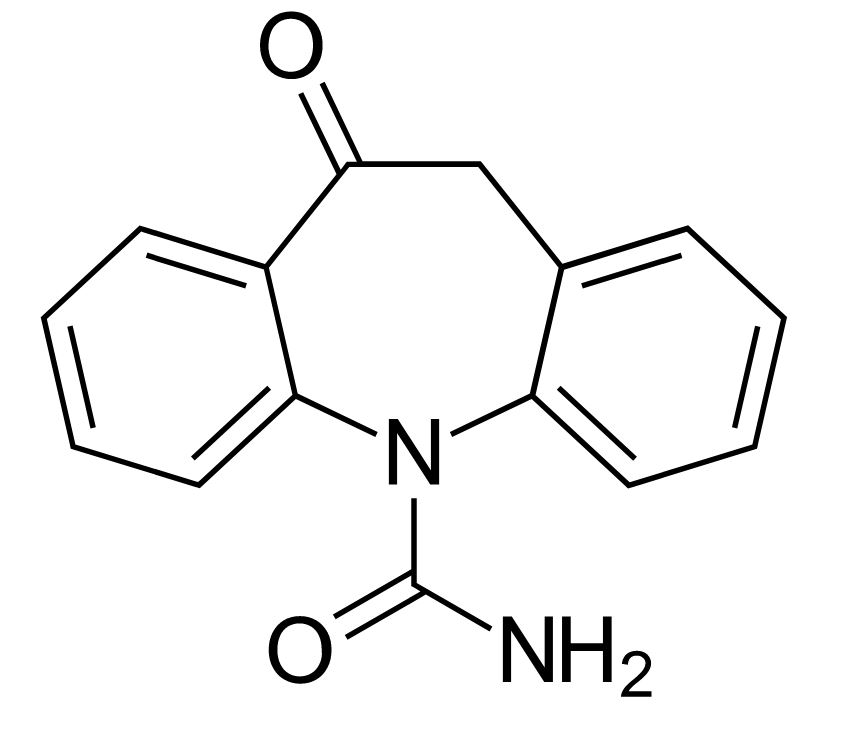
Chemical structure of oxcarbazepine
Image: “Oxcarbazepine” by Harbin. License: Public Domain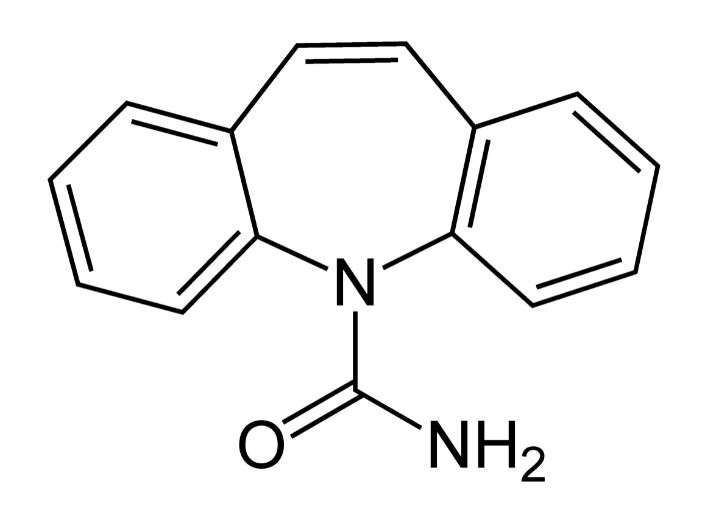
Chemical structure of carbamazepine
Image: “Carbamazepine Structural Formulae” by Jü. License: Public DomainOxcarbazepine Oxcarbazepine A carbamazepine derivative that acts as a voltage-gated sodium channel blocker. It is used for the treatment of partial seizures with or without secondary generalization. It is also an inducer of cytochrome p-450 cyp3a4. First-Generation Anticonvulsant Drugs is indicated in focal (partial)-onset seizures Seizures A seizure is abnormal electrical activity of the neurons in the cerebral cortex that can manifest in numerous ways depending on the region of the brain affected. Seizures consist of a sudden imbalance that occurs between the excitatory and inhibitory signals in cortical neurons, creating a net excitation. The 2 major classes of seizures are focal and generalized. Seizures.
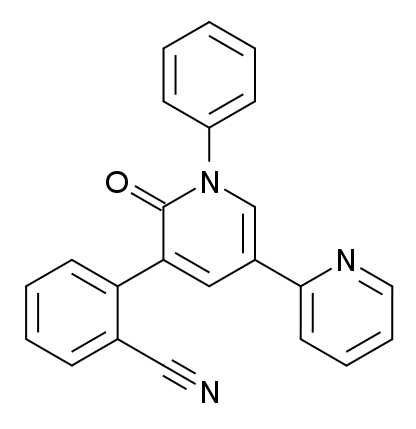
Chemical structure of perampanel
Image: “Perampanel” by Meodipt. License: Public Domain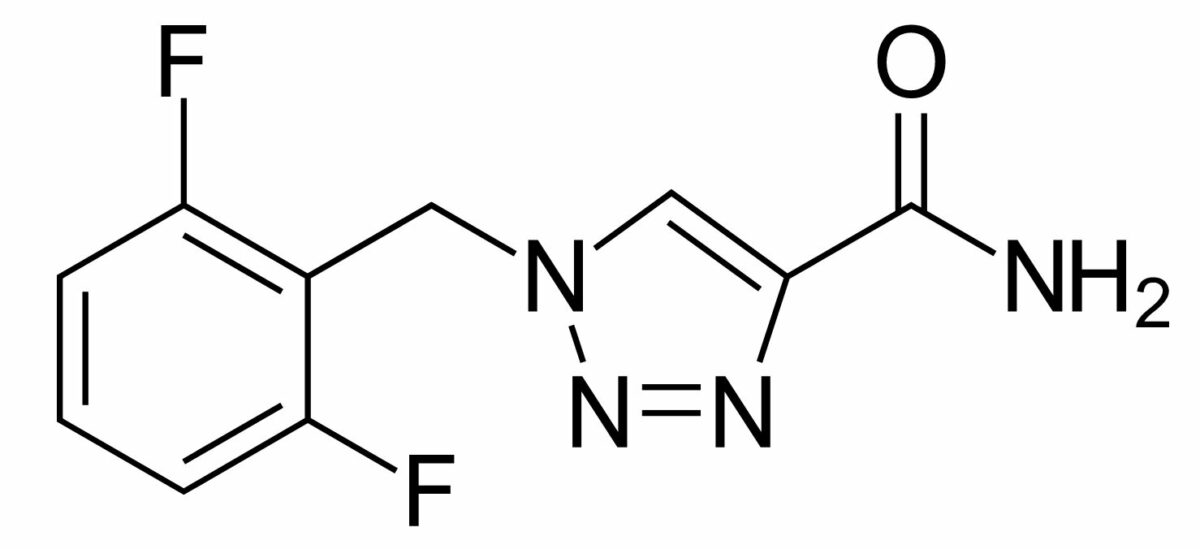
Chemical structure of rufinamide
Image: “Rufinamide” by Vaccinationist. License: Public DomainRufinamide is indicated in the adjunctive treatment of Lennox-Gestaut syndrome.
Stiripentol is indicated in the management of Dravet syndrome (previously known as severe myoclonic epilepsy Epilepsy Epilepsy is a chronic brain disorder marked by recurrent and unprovoked seizures. These seizures can be classified as focal or generalized and idiopathic or secondary to another condition. Clinical presentation correlates to the classification of the epileptic disorder. Epilepsy in infancy).
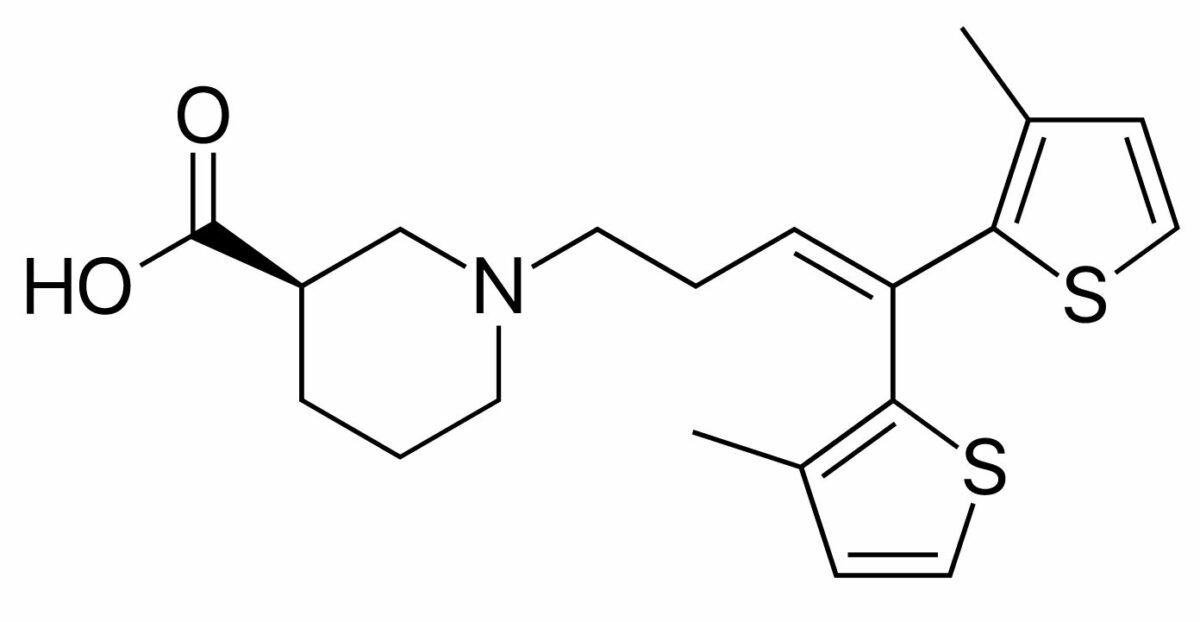
Chemical structure of tiagabine
Image: “Tiagabine” by Fvasconcellos. License: Public DomainTiagabine is indicated for the adjunctive treatment of focal (partial) seizures Seizures A seizure is abnormal electrical activity of the neurons in the cerebral cortex that can manifest in numerous ways depending on the region of the brain affected. Seizures consist of a sudden imbalance that occurs between the excitatory and inhibitory signals in cortical neurons, creating a net excitation. The 2 major classes of seizures are focal and generalized. Seizures.
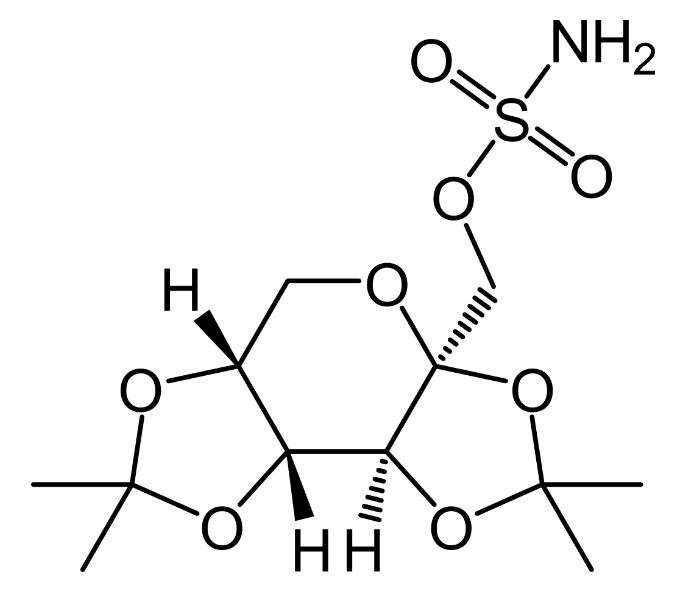
Chemical structure of topiramate
Image: “Topiramate” by UnicornFightCluba. License: Public Domain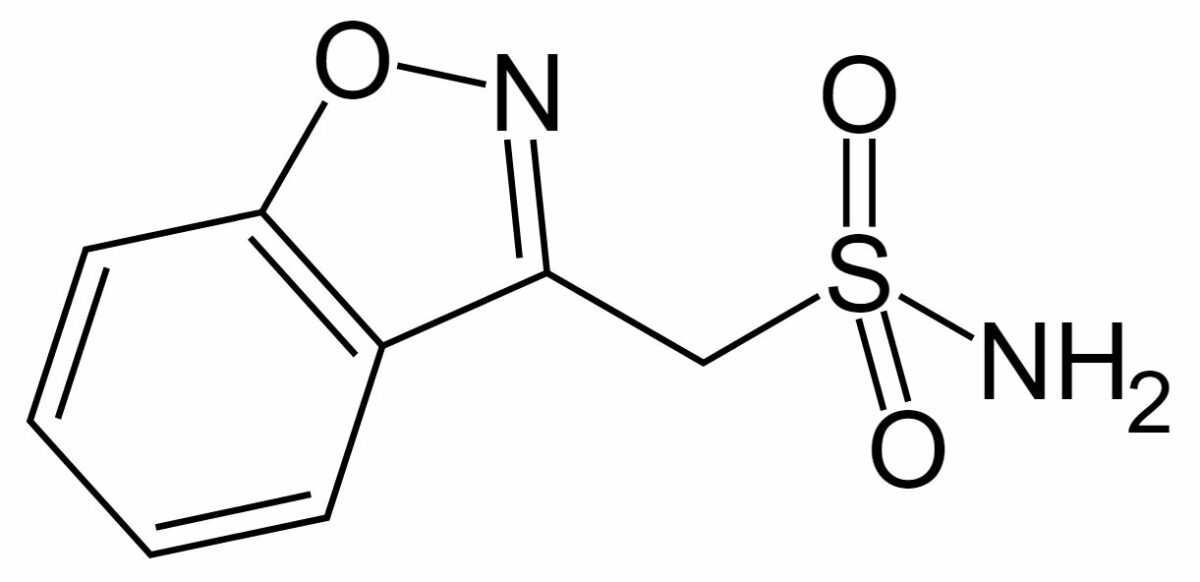
Chemical structure of zonisamide
Image: “Zonisamide” by Fvasconcellos. License: Public DomainZonisamide is indicated for the adjunctive therapy of focal (partial) seizures Seizures A seizure is abnormal electrical activity of the neurons in the cerebral cortex that can manifest in numerous ways depending on the region of the brain affected. Seizures consist of a sudden imbalance that occurs between the excitatory and inhibitory signals in cortical neurons, creating a net excitation. The 2 major classes of seizures are focal and generalized. Seizures.
| Site/factor affected | Main mechanism of action | Drugs |
|---|---|---|
| Na+ channels Channels The Cell: Cell Membrane | Inhibition of voltage-sensitive Na+ channels Channels The Cell: Cell Membrane |
|
| Enhancement of slow inactivation |
|
|
| Calcium Calcium A basic element found in nearly all tissues. It is a member of the alkaline earth family of metals with the atomic symbol ca, atomic number 20, and atomic weight 40. Calcium is the most abundant mineral in the body and combines with phosphorus to form calcium phosphate in the bones and teeth. It is essential for the normal functioning of nerves and muscles and plays a role in blood coagulation (as factor IV) and in many enzymatic processes. Electrolytes channels Channels The Cell: Cell Membrane | Blocking of T-type calcium Calcium A basic element found in nearly all tissues. It is a member of the alkaline earth family of metals with the atomic symbol ca, atomic number 20, and atomic weight 40. Calcium is the most abundant mineral in the body and combines with phosphorus to form calcium phosphate in the bones and teeth. It is essential for the normal functioning of nerves and muscles and plays a role in blood coagulation (as factor IV) and in many enzymatic processes. Electrolytes channels Channels The Cell: Cell Membrane | Zonisamide* |
| Modulation of calcium Calcium A basic element found in nearly all tissues. It is a member of the alkaline earth family of metals with the atomic symbol ca, atomic number 20, and atomic weight 40. Calcium is the most abundant mineral in the body and combines with phosphorus to form calcium phosphate in the bones and teeth. It is essential for the normal functioning of nerves and muscles and plays a role in blood coagulation (as factor IV) and in many enzymatic processes. Electrolytes channels Channels The Cell: Cell Membrane (α2δ subunit) |
|
|
| GABA GABA The most common inhibitory neurotransmitter in the central nervous system. Receptors and Neurotransmitters of the CNS | GABA GABA The most common inhibitory neurotransmitter in the central nervous system. Receptors and Neurotransmitters of the CNS reuptake inhibitors |
|
| Stimulation of GABA GABA The most common inhibitory neurotransmitter in the central nervous system. Receptors and Neurotransmitters of the CNS receptors Receptors Receptors are proteins located either on the surface of or within a cell that can bind to signaling molecules known as ligands (e.g., hormones) and cause some type of response within the cell. Receptors |
|
|
| Glutamate Glutamate Derivatives of glutamic acid. Included under this heading are a broad variety of acid forms, salts, esters, and amides that contain the 2-aminopentanedioic acid structure. Synthesis of Nonessential Amino Acids | Glutamate Glutamate Derivatives of glutamic acid. Included under this heading are a broad variety of acid forms, salts, esters, and amides that contain the 2-aminopentanedioic acid structure. Synthesis of Nonessential Amino Acids receptor Receptor Receptors are proteins located either on the surface of or within a cell that can bind to signaling molecules known as ligands (e.g., hormones) and cause some type of response within the cell. Receptors antagonists |
|
| ↓ Glutamate Glutamate Derivatives of glutamic acid. Included under this heading are a broad variety of acid forms, salts, esters, and amides that contain the 2-aminopentanedioic acid structure. Synthesis of Nonessential Amino Acids release | Lamotrigine* | |
| Neurotransmitter release | Synaptic vesicle Vesicle Primary Skin Lesions 2A modulators |
|
| Indications | Specific conditions | Drugs |
|---|---|---|
| Seizure related | Focal (partial) seizures Seizures A seizure is abnormal electrical activity of the neurons in the cerebral cortex that can manifest in numerous ways depending on the region of the brain affected. Seizures consist of a sudden imbalance that occurs between the excitatory and inhibitory signals in cortical neurons, creating a net excitation. The 2 major classes of seizures are focal and generalized. Seizures |
|
| Primary generalized tonic-clonic seizures Generalized tonic-clonic seizures A generalized seizure disorder characterized by recurrent major motor seizures. The initial brief tonic phase is marked by trunk flexion followed by diffuse extension of the trunk and extremities. The clonic phase features rhythmic flexor contractions of the trunk and limbs, pupillary dilation, elevations of blood pressure and pulse, urinary incontinence, and tongue biting. This is followed by a profound state of depressed consciousness (post-ictal state) which gradually improves over minutes to hours. The disorder may be cryptogenic, familial, or symptomatic (caused by an identified disease process). Epilepsy |
|
|
| Lennox-Gastaut syndrome Lennox-Gastaut Syndrome Epilepsy |
|
|
| Juvenile myoclonic epilepsy Juvenile myoclonic epilepsy A disorder characterized by the onset of myoclonus in adolescence, a marked increase in the incidence of absence seizures, and generalized major motor seizures. The myoclonic episodes tend to occur shortly after awakening. Seizures tend to be aggravated by sleep deprivation and alcohol consumption. Hereditary and sporadic forms have been identified. Epilepsy | Levetiracetam | |
| Infantile spasms Spasms An involuntary contraction of a muscle or group of muscles. Spasms may involve skeletal muscle or smooth muscle. Ion Channel Myopathy and refractory complex partial seizures Seizures A seizure is abnormal electrical activity of the neurons in the cerebral cortex that can manifest in numerous ways depending on the region of the brain affected. Seizures consist of a sudden imbalance that occurs between the excitatory and inhibitory signals in cortical neurons, creating a net excitation. The 2 major classes of seizures are focal and generalized. Seizures | Vigabatrin | |
| Dravet syndrome | Stiripentol | |
| Seizure unrelated |
|
Pregabalin |
| Postherpetic neuralgia Postherpetic neuralgia Pain in nerves, frequently involving facial skin, resulting from the activation the latent varicella-zoster virus. The two forms of the condition preceding the pain are herpes zoster oticus; and herpes zoster ophthalmicus. Following the healing of the rashes and blisters, the pain sometimes persists. Herpes Zoster (Shingles) |
|
|
| Bipolar Bipolar Nervous System: Histology disorder | Lamotrigine | |
| Migraine Migraine Migraine headache is a primary headache disorder and is among the most prevalent disorders in the world. Migraine is characterized by episodic, moderate to severe headaches that may be associated with increased sensitivity to light and sound, as well as nausea and/or vomiting. Migraine Headache prophylaxis Prophylaxis Cephalosporins | Topiramate |