Class 3 antiarrhythmics are drugs that block cardiac tissue K channels Channels The Cell: Cell Membrane. The medications in this class include amiodarone Amiodarone An antianginal and class III antiarrhythmic drug. It increases the duration of ventricular and atrial muscle action by inhibiting potassium channels and voltage-gated sodium channels. There is a resulting decrease in heart rate and in vascular resistance. Pulmonary Fibrosis, dronedarone, sotalol, ibutilide, dofetilide, and bretylium. The main mechanism of action includes blocking the cardiac K channels Channels The Cell: Cell Membrane to prolong repolarization Repolarization Membrane Potential. However, some medications in this class also exert effects on Na channels Channels The Cell: Cell Membrane, calcium Calcium A basic element found in nearly all tissues. It is a member of the alkaline earth family of metals with the atomic symbol ca, atomic number 20, and atomic weight 40. Calcium is the most abundant mineral in the body and combines with phosphorus to form calcium phosphate in the bones and teeth. It is essential for the normal functioning of nerves and muscles and plays a role in blood coagulation (as factor IV) and in many enzymatic processes. Electrolytes channels Channels The Cell: Cell Membrane, and adrenergic receptors Receptors Receptors are proteins located either on the surface of or within a cell that can bind to signaling molecules known as ligands (e.g., hormones) and cause some type of response within the cell. Receptors. Indications vary among the medications, but include both atrial and ventricular arrhythmias. Because these medications prolong the QT interval QT interval Electrocardiogram (ECG), torsades de pointes Torsades de pointes A malignant form of polymorphic ventricular tachycardia that is characterized by heart rate between 200 and 250 beats per minute, and QRS complexes with changing amplitude and twisting of the points. The term also describes the syndrome of tachycardia with prolonged ventricular repolarization, long qt intervals exceeding 500 milliseconds or bradycardia. Torsades de pointes may be self-limited or may progress to ventricular fibrillation. Ventricular Tachycardia is a potential complication of therapy.
Last updated: Sep 1, 2022
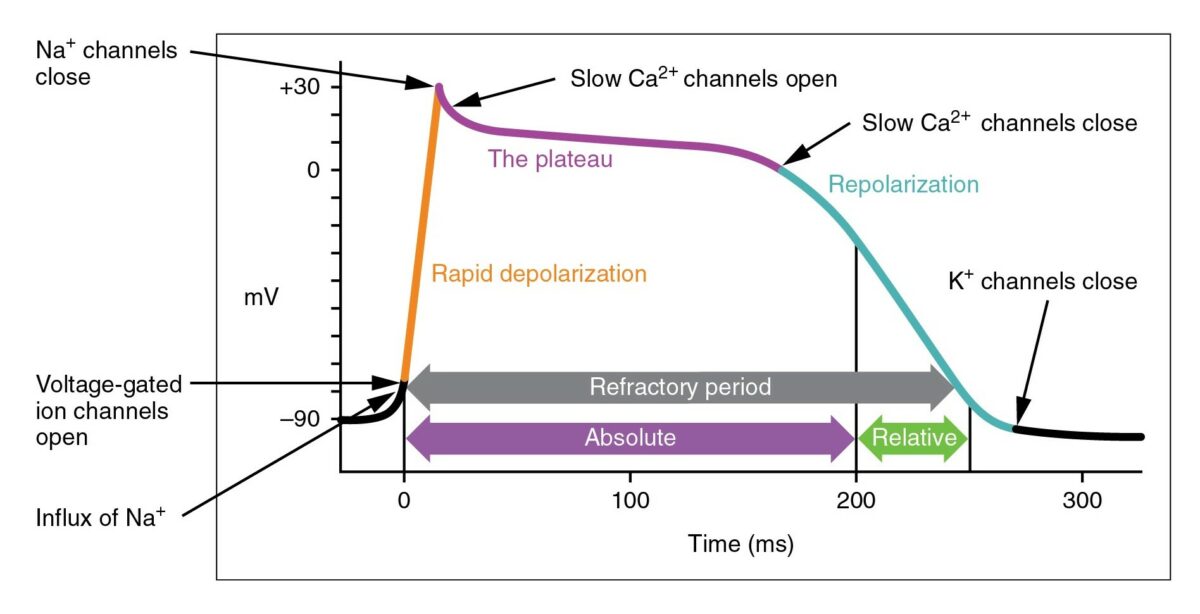
The cardiac action potential
Image: “Action Potential Heart Contraction, Illustration from Anatomy & Physiology” by OpenStax College. License: CC BY 3.0, cropped by Lecturio.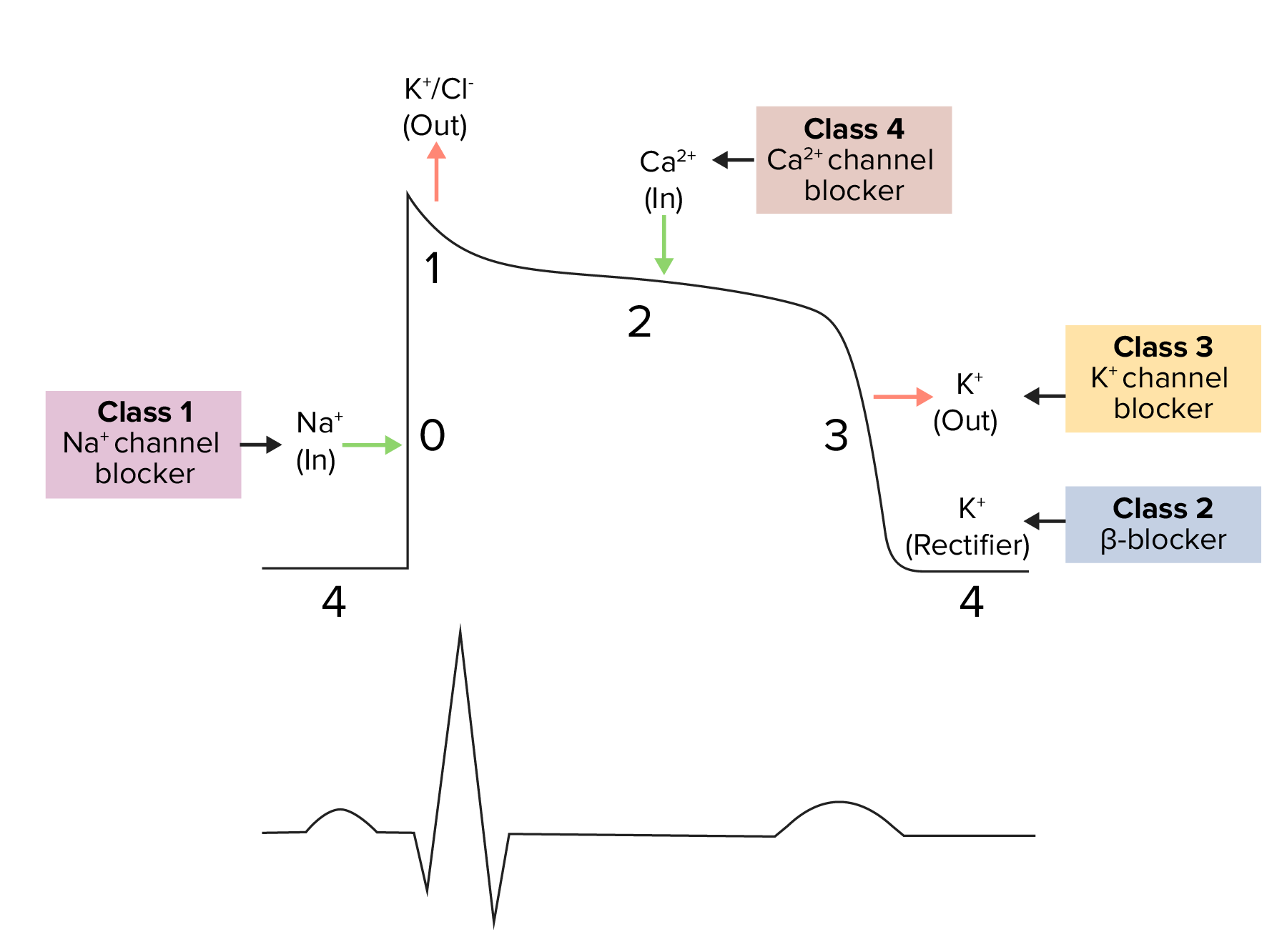
Diagram demonstrating a cardiac action potential and the phases of action for different antiarrhythmic drug classes:
The cycle starts with phase 4, the resting potential. Phase 0 is when rapid depolarization happens due to an influx of sodium ions into the cell. Repolarization follows, with an efflux of potassium through fast potassium channels in phase 1, calcium influx in phase 2, and efflux of potassium through delayed potassium channels in phase 3. Potassium channel blockers typically affect phase 3.
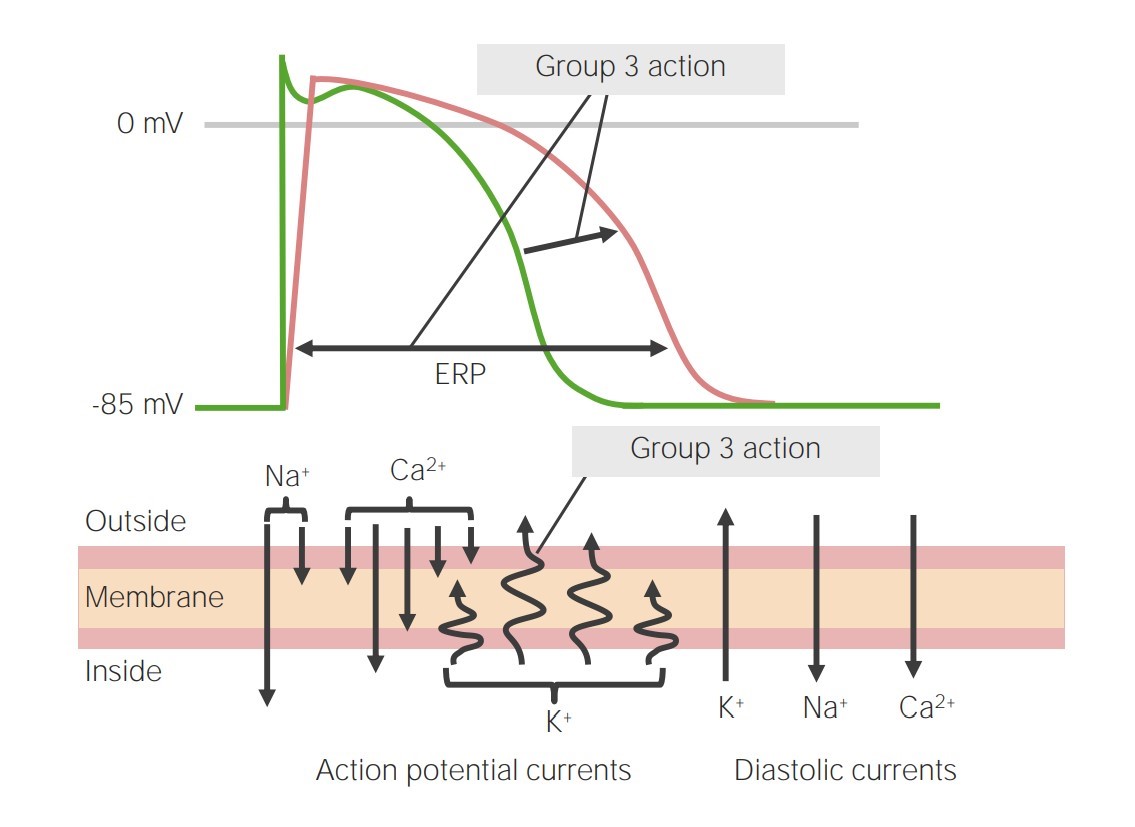
Image representing the action of class 3 antiarrhythmics on phase 3 of the action potential:
By blocking the potassium channels, phase 3 is prolonged, leading to an increase in the effective refractory period (ERP).
Class 3 antiarrhythmics include a variety of medications that vary in K channel selectivity and other antiarrhythmic effects:
Adverse effects of amiodarone Amiodarone An antianginal and class III antiarrhythmic drug. It increases the duration of ventricular and atrial muscle action by inhibiting potassium channels and voltage-gated sodium channels. There is a resulting decrease in heart rate and in vascular resistance. Pulmonary Fibrosis are seen mainly in long-term oral therapy (rather than short-term IV therapy).
Cardiovascular effects:
Pulmonary toxicity Toxicity Dosage Calculation:
Thyroid Thyroid The thyroid gland is one of the largest endocrine glands in the human body. The thyroid gland is a highly vascular, brownish-red gland located in the visceral compartment of the anterior region of the neck. Thyroid Gland: Anatomy dysfunction:
Hepatotoxicity Hepatotoxicity Acetaminophen:
Neurologic toxicity Toxicity Dosage Calculation:
Ocular effects:
Cutaneous reactions:
Ibutilide is used to pharmacologically convert AF AF Atrial fibrillation (AF or Afib) is a supraventricular tachyarrhythmia and the most common kind of arrhythmia. It is caused by rapid, uncontrolled atrial contractions and uncoordinated ventricular responses. Atrial Fibrillation or atrial flutter Atrial flutter Atrial flutter is a regular supraventricular tachycardia characterized by an atrial heart rate between 240/min and 340/min (typically 300/min), atrioventricular (AV) node conduction block, and a “sawtooth” pattern on an electrocardiogram (ECG). Atrial Flutter to sinus rhythm Sinus rhythm A heart rate and rhythm driven by the regular firing of the SA node (60–100 beats per minute) Cardiac Physiology.
Increased QT prolongation can occur with:
Dofetilide is used for cardioversion Cardioversion Atrial Fibrillation and maintenance of sinus rhythm Sinus rhythm A heart rate and rhythm driven by the regular firing of the SA node (60–100 beats per minute) Cardiac Physiology in atrial fibrillation Atrial fibrillation Atrial fibrillation (AF or Afib) is a supraventricular tachyarrhythmia and the most common kind of arrhythmia. It is caused by rapid, uncontrolled atrial contractions and uncoordinated ventricular responses. Atrial Fibrillation and atrial flutter Atrial flutter Atrial flutter is a regular supraventricular tachycardia characterized by an atrial heart rate between 240/min and 340/min (typically 300/min), atrioventricular (AV) node conduction block, and a “sawtooth” pattern on an electrocardiogram (ECG). Atrial Flutter.
Bretylium is used for ventricular arrhythmias:
The following table compares antiarrhythmic classes 1–4. Class 5 is not included owing to the varied mechanisms of action and effects.
| Class | Mechanism of action | Effects | Arrhythmia indications | |
|---|---|---|---|---|
| 1 | 1A |
|
|
|
| 1B | Ventricular | |||
| 1C | Mostly atrial | |||
| 2 |
|
|
Atrial and ventricular | |
| 3 |
|
|
Atrial and ventricular | |
| 4 |
|
|
Atrial | |