Anticoagulants are drugs that retard or interrupt the coagulation cascade Coagulation cascade The coagulation cascade is a series of reactions that ultimately generates a strong, cross-linked fibrin clot. Hemostasis. The primary classes of available anticoagulants include heparins, vitamin K-dependent antagonists (e.g., warfarin), direct thrombin Thrombin An enzyme formed from prothrombin that converts fibrinogen to fibrin. Hemostasis inhibitors, and factor Xa inhibitors. Anticoagulants are used in the treatment and prevention of thrombotic and embolic diseases including cardioembolic ischemic stroke Ischemic Stroke An ischemic stroke (also known as cerebrovascular accident) is an acute neurologic injury that occurs as a result of brain ischemia; this condition may be due to cerebral blood vessel occlusion by thrombosis or embolism, or rarely due to systemic hypoperfusion. Ischemic Stroke, acute coronary syndrome, and venous thromboembolism Thromboembolism Obstruction of a blood vessel (embolism) by a blood clot (thrombus) in the blood stream. Systemic Lupus Erythematosus, among other conditions. Patients Patients Individuals participating in the health care system for the purpose of receiving therapeutic, diagnostic, or preventive procedures. Clinician–Patient Relationship with atrial fibrillation Atrial fibrillation Atrial fibrillation (AF or Afib) is a supraventricular tachyarrhythmia and the most common kind of arrhythmia. It is caused by rapid, uncontrolled atrial contractions and uncoordinated ventricular responses. Atrial Fibrillation or thrombophilias Thrombophilias Hypercoagulable states (also referred to as thrombophilias) are a group of hematologic diseases defined by an increased risk of clot formation (i.e., thrombosis) due to either an increase in procoagulants, a decrease in anticoagulants, or a decrease in fibrinolysis. Hypercoagulable States may require indefinite or lifelong anticoagulation Anticoagulation Pulmonary Hypertension Drugs. Accordingly, the route of administration, drug interactions, pharmacokinetics Pharmacokinetics Pharmacokinetics is the science that analyzes how the human body interacts with a drug. Pharmacokinetics examines how the drug is absorbed, distributed, metabolized, and excreted by the body. Pharmacokinetics and Pharmacodynamics, and availability of reversal factors should be considered while selecting the anticoagulant therapy.
Last updated: Jan 17, 2024
Anticoagulants are a category of drugs that inhibit the coagulation cascade Coagulation cascade The coagulation cascade is a series of reactions that ultimately generates a strong, cross-linked fibrin clot. Hemostasis.
Anticoagulants are indicated in the treatment and prophylaxis Prophylaxis Cephalosporins of thrombotic events including:
There are several primary classes of anticoagulants:
The coagulation cascade Coagulation cascade The coagulation cascade is a series of reactions that ultimately generates a strong, cross-linked fibrin clot. Hemostasis is a series of reactions that ultimately generates a strong, cross-linked fibrin Fibrin A protein derived from fibrinogen in the presence of thrombin, which forms part of the blood clot. Rapidly Progressive Glomerulonephritis clot. This cascade is also known as secondary hemostasis Secondary hemostasis The coagulation cascade is a series of reactions that ultimately generates a strong, cross-linked fibrin clot. Hemostasis.
Coagulation factors Coagulation factors Endogenous substances, usually proteins, that are involved in the blood coagulation process. Hemostasis:
A number of coagulation factors Coagulation factors Endogenous substances, usually proteins, that are involved in the blood coagulation process. Hemostasis undergo sequential Sequential Computed Tomography (CT) activation down 1 of 2 pathways:
Common pathway Common pathway Hemostasis:
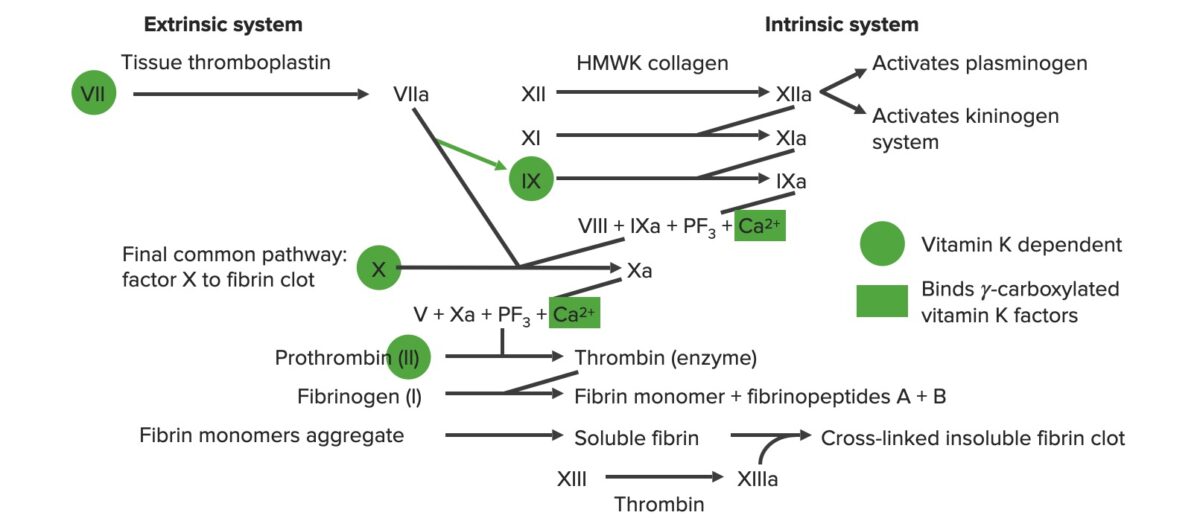
Overview of the coagulation cascade
a: activated form
PF3: platelet factor 3 (phospholipids)
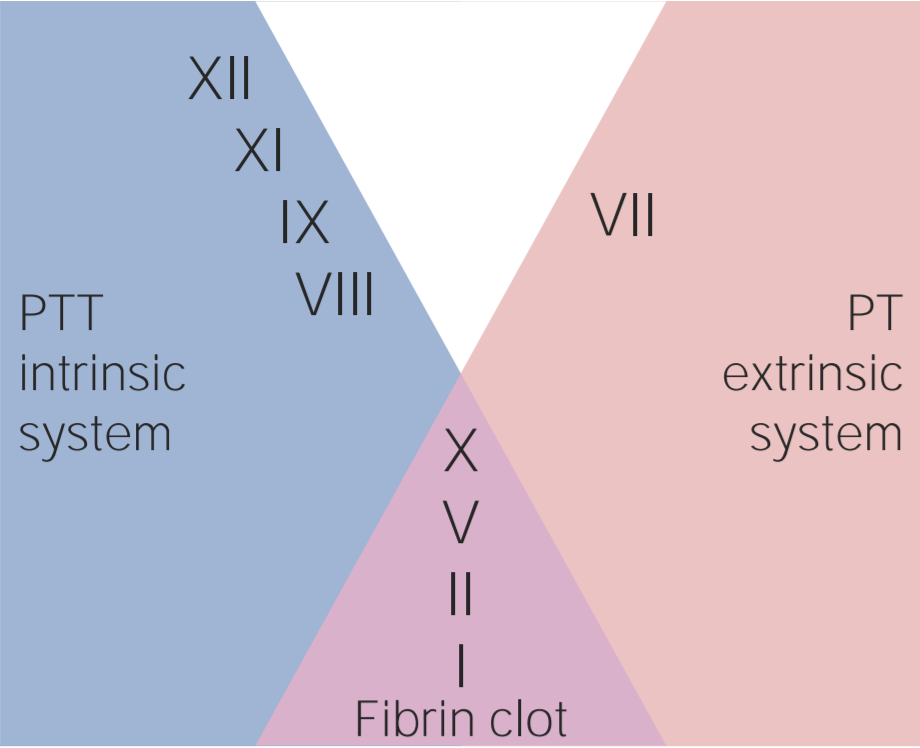
Factors involved in the intrinsic, extrinsic, and common pathways
Image by Lecturio.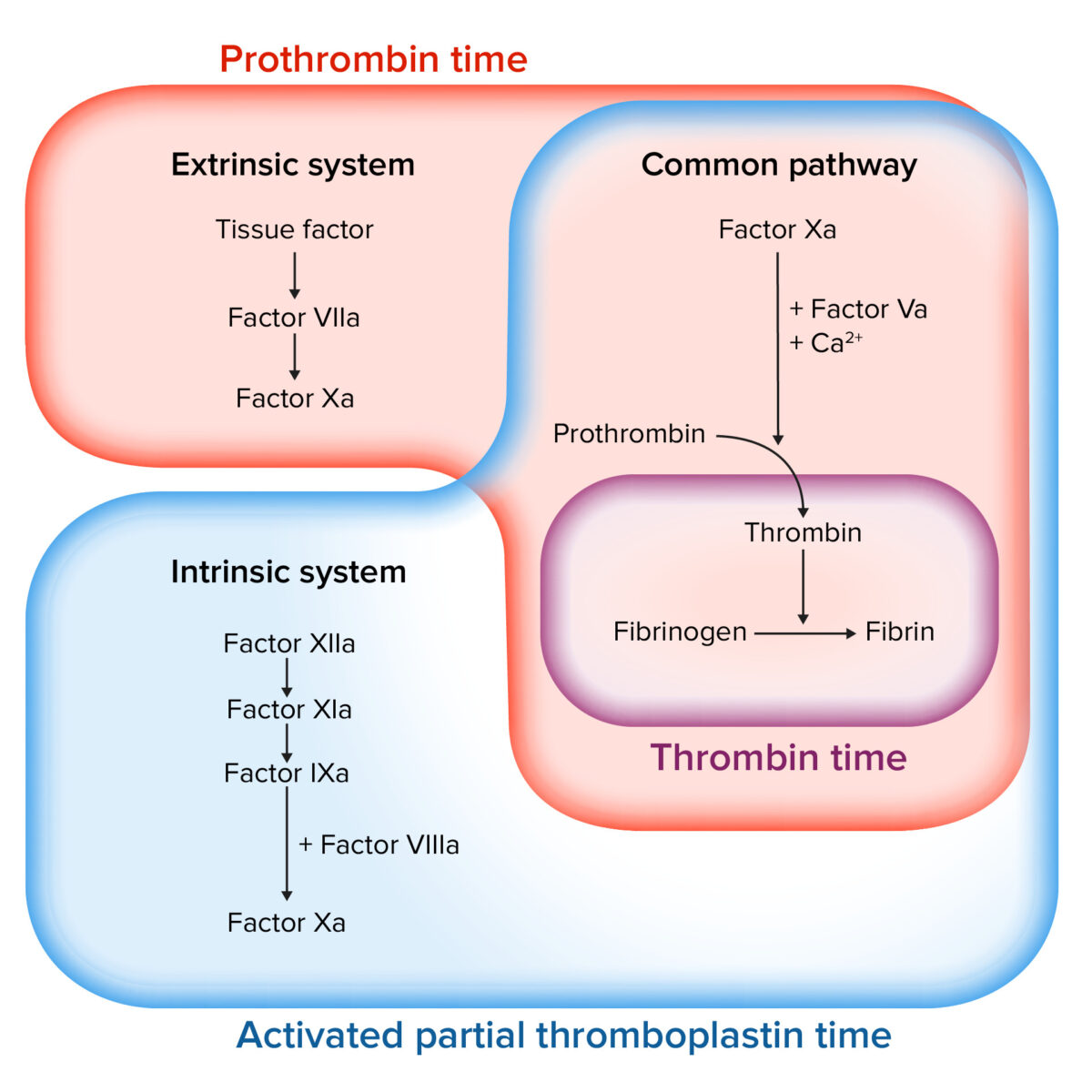
Assessing the coagulation cascade
Image by Lecturio.Natural heparins are a group of large, endogenously produced polysaccharides Polysaccharides Basics of Carbohydrates of varying sizes that are not completely understood. Heparins have anticoagulant, anti-inflammatory, and possibly anti-angiogenic effects.
| UFH | LMWH | |
|---|---|---|
| Agents | UFH |
|
| Mechanism of action |
Binds to and potentiates antithrombin
|
|
| Physiologic effects | Inactivation of Factor Xa and thrombin Thrombin An enzyme formed from prothrombin that converts fibrinogen to fibrin. Hemostasis | Inactivation of Factor Xa and, to a much lesser extent, thrombin Thrombin An enzyme formed from prothrombin that converts fibrinogen to fibrin. Hemostasis |
| Absorption Absorption Absorption involves the uptake of nutrient molecules and their transfer from the lumen of the GI tract across the enterocytes and into the interstitial space, where they can be taken up in the venous or lymphatic circulation. Digestion and Absorption |
Administered IV (rarely SC):
|
|
| Distribution | Vd = ~ 35 mL/kg | Vd = 4.3 L |
| Metabolism |
|
Liver Liver The liver is the largest gland in the human body. The liver is found in the superior right quadrant of the abdomen and weighs approximately 1.5 kilograms. Its main functions are detoxification, metabolism, nutrient storage (e.g., iron and vitamins), synthesis of coagulation factors, formation of bile, filtration, and storage of blood. Liver: Anatomy |
| Elimination Elimination The initial damage and destruction of tumor cells by innate and adaptive immunity. Completion of the phase means no cancer growth. Cancer Immunotherapy |
|
|
| Monitoring |
|
|
| Reversal agent | Protamine sulfate | Protamine sulfate |
| Complications |
|
Lower risks of HITT and osteoporosis Osteoporosis Osteoporosis refers to a decrease in bone mass and density leading to an increased number of fractures. There are 2 forms of osteoporosis: primary, which is commonly postmenopausal or senile; and secondary, which is a manifestation of immobilization, underlying medical disorders, or long-term use of certain medications. Osteoporosis than UFH |
| Specific contraindications Contraindications A condition or factor associated with a recipient that makes the use of a drug, procedure, or physical agent improper or inadvisable. Contraindications may be absolute (life threatening) or relative (higher risk of complications in which benefits may outweigh risks). Noninvasive Ventilation |
|
|
| Notes |
|
|
| Mechanism of action |
|
|---|---|
| Absorption Absorption Absorption involves the uptake of nutrient molecules and their transfer from the lumen of the GI tract across the enterocytes and into the interstitial space, where they can be taken up in the venous or lymphatic circulation. Digestion and Absorption |
|
| Metabolism |
Metabolized in
liver
Liver
The liver is the largest gland in the human body. The liver is found in the superior right quadrant of the abdomen and weighs approximately 1.5 kilograms. Its main functions are detoxification, metabolism, nutrient storage (e.g., iron and vitamins), synthesis of coagulation factors, formation of bile, filtration, and storage of blood.
Liver: Anatomy:
|
| Distribution |
|
| Elimination Elimination The initial damage and destruction of tumor cells by innate and adaptive immunity. Completion of the phase means no cancer growth. Cancer Immunotherapy |
|
| Monitoring |
|
| Reversal agent/ antidote Antidote An antidote is a substance that counteracts poisoning or toxicity. Substances that can cause poisoning include heavy metals (from occupation, treatments, or diet), alcohols, environmental toxins, and medications. Antidotes of Common Poisonings | Vitamin K Vitamin K A lipid cofactor that is required for normal blood clotting. Several forms of vitamin K have been identified: vitamin K 1 (phytomenadione) derived from plants, vitamin K 2 (menaquinone) from bacteria, and synthetic naphthoquinone provitamins, vitamin K 3 (menadione). Vitamin k 3 provitamins, after being alkylated in vivo, exhibit the antifibrinolytic activity of vitamin k. Green leafy vegetables, liver, cheese, butter, and egg yolk are good sources of vitamin k. Fat-soluble Vitamins and their Deficiencies (takes several hours for effect) |
| Interactions | Warfarin has numerous drug, herbal, and dietary interactions:
|
| Specific contraindications Contraindications A condition or factor associated with a recipient that makes the use of a drug, procedure, or physical agent improper or inadvisable. Contraindications may be absolute (life threatening) or relative (higher risk of complications in which benefits may outweigh risks). Noninvasive Ventilation | Pregnancy Pregnancy The status during which female mammals carry their developing young (embryos or fetuses) in utero before birth, beginning from fertilization to birth. Pregnancy: Diagnosis, Physiology, and Care (warfarin is teratogenic) |
| Complications |
|
| Notes | Patients Patients Individuals participating in the health care system for the purpose of receiving therapeutic, diagnostic, or preventive procedures. Clinician–Patient Relationship with CYP2C9 variants have ↓ enzyme activity → require ↓ dose |
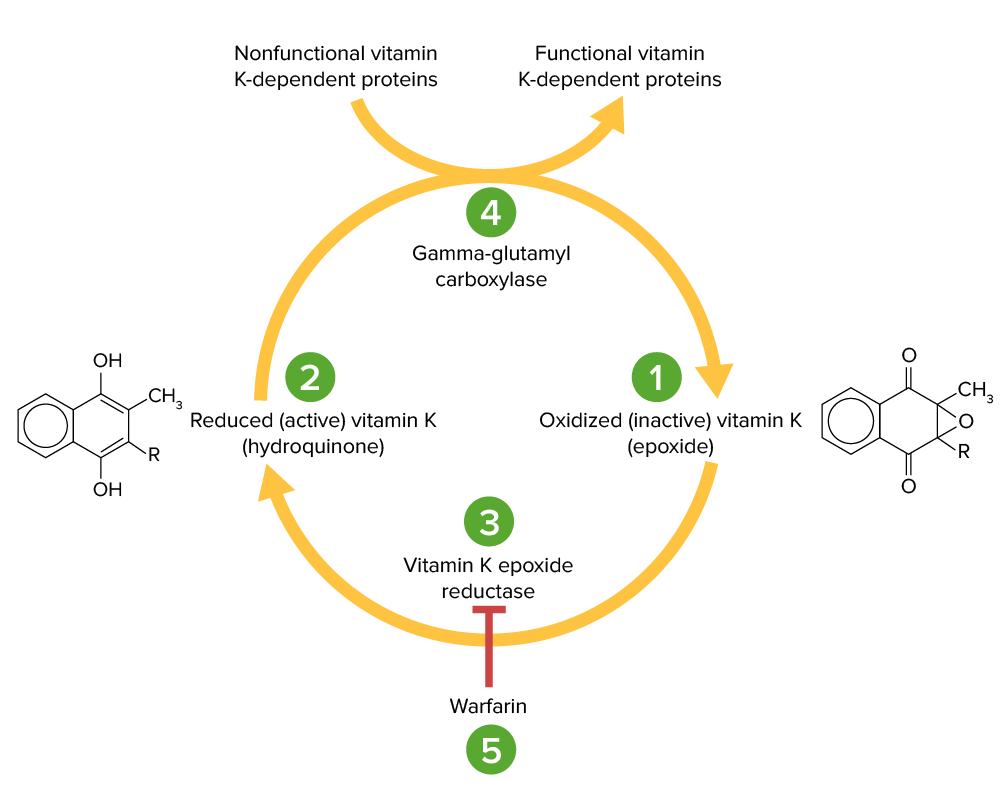
Vitamin K cycle:
Vitamin K epoxide (1) is inactive and converted to its active, reduced form, vitamin K hydroquinone (2), by vitamin K epoxide reductase (VKOR; 3). Vitamin K hydroquinone is a cofactor in the carboxylation of specific glutamate residues within the vitamin K-dependent proteins (factors II, VII, IX, X, protein C and S), a process which is necessary to activate them. The carboxylation reaction is catalyzed by gamma-glutamyl carboxylase (4). Vitamin K hydroquinone is oxidized to the epoxide form when it acts as a cofactor, but is then recycled back to the hydroquinone form by VKOR. Warfarin inhibits VKOR (5) so that vitamin K cannot be recycled from its oxidized form to the reduced form. Thus, vitamin K-dependent proteins cannot be activated.
| Oral agents (DOAC) | Parenteral agents | |
|---|---|---|
| Agents | Dabigatran (Pradaxa®) |
|
| Mechanism of action | Binds to and functionally inhibits thrombin Thrombin An enzyme formed from prothrombin that converts fibrinogen to fibrin. Hemostasis in both serum and clots | |
| Absorption Absorption Absorption involves the uptake of nutrient molecules and their transfer from the lumen of the GI tract across the enterocytes and into the interstitial space, where they can be taken up in the venous or lymphatic circulation. Digestion and Absorption |
|
Onset of action: immediate |
| Distribution |
|
Bivalirudin:
|
| Metabolism | Liver Liver The liver is the largest gland in the human body. The liver is found in the superior right quadrant of the abdomen and weighs approximately 1.5 kilograms. Its main functions are detoxification, metabolism, nutrient storage (e.g., iron and vitamins), synthesis of coagulation factors, formation of bile, filtration, and storage of blood. Liver: Anatomy |
|
| Elimination Elimination The initial damage and destruction of tumor cells by innate and adaptive immunity. Completion of the phase means no cancer growth. Cancer Immunotherapy |
|
|
| Monitoring | aPTT | ACT |
| Antidote Antidote An antidote is a substance that counteracts poisoning or toxicity. Substances that can cause poisoning include heavy metals (from occupation, treatments, or diet), alcohols, environmental toxins, and medications. Antidotes of Common Poisonings | Idarucizumab (Praxbind®) | None |
| Specific contraindications Contraindications A condition or factor associated with a recipient that makes the use of a drug, procedure, or physical agent improper or inadvisable. Contraindications may be absolute (life threatening) or relative (higher risk of complications in which benefits may outweigh risks). Noninvasive Ventilation | None (beyond general contraindications Contraindications A condition or factor associated with a recipient that makes the use of a drug, procedure, or physical agent improper or inadvisable. Contraindications may be absolute (life threatening) or relative (higher risk of complications in which benefits may outweigh risks). Noninvasive Ventilation listed above) | |
| Notes | Often used as an alternative in patients Patients Individuals participating in the health care system for the purpose of receiving therapeutic, diagnostic, or preventive procedures. Clinician–Patient Relationship with a history of HITT | |
| Direct factor Xa inhibitors (DOACs) | Indirect factor Xa inhibitors | |
|---|---|---|
| Agents |
|
|
| Mechanism of action | Directly binds to and inhibits factor Xa |
|
| Absorption Absorption Absorption involves the uptake of nutrient molecules and their transfer from the lumen of the GI tract across the enterocytes and into the interstitial space, where they can be taken up in the venous or lymphatic circulation. Digestion and Absorption |
|
|
| Distribution |
Rivaroxaban:
|
|
| Metabolism | Primarily metabolized in the liver Liver The liver is the largest gland in the human body. The liver is found in the superior right quadrant of the abdomen and weighs approximately 1.5 kilograms. Its main functions are detoxification, metabolism, nutrient storage (e.g., iron and vitamins), synthesis of coagulation factors, formation of bile, filtration, and storage of blood. Liver: Anatomy by CYP3A4 CYP3A4 Class 3 Antiarrhythmic Drugs (Potassium Channel Blockers) | Eliminated unchanged |
| Elimination Elimination The initial damage and destruction of tumor cells by innate and adaptive immunity. Completion of the phase means no cancer growth. Cancer Immunotherapy |
|
|
| Monitoring |
|
|
| Antidote Antidote An antidote is a substance that counteracts poisoning or toxicity. Substances that can cause poisoning include heavy metals (from occupation, treatments, or diet), alcohols, environmental toxins, and medications. Antidotes of Common Poisonings | Andexanet alfa | None |
| Specific contraindications Contraindications A condition or factor associated with a recipient that makes the use of a drug, procedure, or physical agent improper or inadvisable. Contraindications may be absolute (life threatening) or relative (higher risk of complications in which benefits may outweigh risks). Noninvasive Ventilation | None (beyond general contraindications Contraindications A condition or factor associated with a recipient that makes the use of a drug, procedure, or physical agent improper or inadvisable. Contraindications may be absolute (life threatening) or relative (higher risk of complications in which benefits may outweigh risks). Noninvasive Ventilation listed above) | Thrombocytopenia Thrombocytopenia Thrombocytopenia occurs when the platelet count is < 150,000 per microliter. The normal range for platelets is usually 150,000-450,000/µL of whole blood. Thrombocytopenia can be a result of decreased production, increased destruction, or splenic sequestration of platelets. Patients are often asymptomatic until platelet counts are < 50,000/µL. Thrombocytopenia associated with a positive antiplatelet antibody test |
| Notes | A synthetic pentasaccharide with a functional site similar to heparin |
Factors to consider prior to reversing an anticoagulant:
| Medication | Time prior to the procedure for which the medication should be stopped |
|---|---|
| Warfarin | 5 days |
| Heparin | 4 hours |
| LMWH |
|
| DOACs: Dabigatran, rivaroxaban, apixaban, edoxaban |
|
| Medication | Reversal agent |
|---|---|
| Warfarin |
|
| Heparin and LMWHs | Protamine sulfate |
| Dabigatran | Idarucizumab (Praxbind®) |
| Argatroban, bivalirudin | None |
| Apixaban, rivaroxaban | Andexanet alfa |
| Fondaparinux | None, consider recombinant activated factor VII Factor VII Heat- and storage-stable plasma protein that is activated by tissue thromboplastin to form factor viia in the extrinsic pathway of blood coagulation. The activated form then catalyzes the activation of factor X to factor Xa. Hemostasis |
Some of the most common therapeutic uses of anticoagulants include: