Human immunodeficiency Immunodeficiency Chédiak-Higashi Syndrome virus Virus Viruses are infectious, obligate intracellular parasites composed of a nucleic acid core surrounded by a protein capsid. Viruses can be either naked (non-enveloped) or enveloped. The classification of viruses is complex and based on many factors, including type and structure of the nucleoid and capsid, the presence of an envelope, the replication cycle, and the host range. Virology ( HIV HIV Anti-HIV Drugs), a single-stranded RNA RNA A polynucleotide consisting essentially of chains with a repeating backbone of phosphate and ribose units to which nitrogenous bases are attached. RNA is unique among biological macromolecules in that it can encode genetic information, serve as an abundant structural component of cells, and also possesses catalytic activity. RNA Types and Structure virus Virus Viruses are infectious, obligate intracellular parasites composed of a nucleic acid core surrounded by a protein capsid. Viruses can be either naked (non-enveloped) or enveloped. The classification of viruses is complex and based on many factors, including type and structure of the nucleoid and capsid, the presence of an envelope, the replication cycle, and the host range. Virology belonging to the Retroviridae Retroviridae The human immunodeficiency virus (HIV) is a species of Lentivirus, a genus of the family Retroviridae, which causes HIV infections and acquired immunodeficiency syndrome (AIDS). The virus has high genetic variability and is divided into 2 major types, HIV type 1 (HIV-1) and HIV type 2 (HIV-2). The human immunodeficiency virus is a single-stranded, positive-sense, enveloped RNA virus, which targets and destroys WBCs, leading to frequent opportunistic infections and, eventually, death. Human Immunodeficiency Virus (HIV) family, is the etiologic agent of acquired immunodeficiency Immunodeficiency Chédiak-Higashi Syndrome syndrome (AIDS). The human immunodeficiency Immunodeficiency Chédiak-Higashi Syndrome virus Virus Viruses are infectious, obligate intracellular parasites composed of a nucleic acid core surrounded by a protein capsid. Viruses can be either naked (non-enveloped) or enveloped. The classification of viruses is complex and based on many factors, including type and structure of the nucleoid and capsid, the presence of an envelope, the replication cycle, and the host range. Virology is a sexually transmitted or blood-borne infection that attacks CD4+ T lymphocyte cells, macrophages Macrophages The relatively long-lived phagocytic cell of mammalian tissues that are derived from blood monocytes. Main types are peritoneal macrophages; alveolar macrophages; histiocytes; kupffer cells of the liver; and osteoclasts. They may further differentiate within chronic inflammatory lesions to epithelioid cells or may fuse to form foreign body giant cells or langhans giant cells. Innate Immunity: Phagocytes and Antigen Presentation, and dendritic cells Dendritic cells Specialized cells of the hematopoietic system that have branch-like extensions. They are found throughout the lymphatic system, and in non-lymphoid tissues such as skin and the epithelia of the intestinal, respiratory, and reproductive tracts. They trap and process antigens, and present them to T-cells, thereby stimulating cell-mediated immunity. They are different from the non-hematopoietic follicular dendritic cells, which have a similar morphology and immune system function, but with respect to humoral immunity (antibody production). Skin: Structure and Functions, leading to eventual immunodeficiency Immunodeficiency Chédiak-Higashi Syndrome. The presentation is marked by constitutional symptoms Constitutional Symptoms Antineutrophil Cytoplasmic Antibody (ANCA)-Associated Vasculitis such as lymphadenopathy Lymphadenopathy Lymphadenopathy is lymph node enlargement (> 1 cm) and is benign and self-limited in most patients. Etiologies include malignancy, infection, and autoimmune disorders, as well as iatrogenic causes such as the use of certain medications. Generalized lymphadenopathy often indicates underlying systemic disease. Lymphadenopathy and fever Fever Fever is defined as a measured body temperature of at least 38°C (100.4°F). Fever is caused by circulating endogenous and/or exogenous pyrogens that increase levels of prostaglandin E2 in the hypothalamus. Fever is commonly associated with chills, rigors, sweating, and flushing of the skin. Fever. Further progression predisposes to opportunistic infections Infections Invasion of the host organism by microorganisms or their toxins or by parasites that can cause pathological conditions or diseases. Chronic Granulomatous Disease and malignancies. Diagnosis is by enzyme immunoassay for HIV-1 and -2. Additional tests include HIV HIV Anti-HIV Drugs viral load, genotyping Genotyping Methods used to determine individuals' specific alleles or snps (single nucleotide polymorphisms). Polymerase Chain Reaction (PCR), and CD4+ T lymphocyte count Lymphocyte count The number of lymphocytes per unit volume of blood. Lymphocytosis to determine therapy and evaluate treatment response and disease progression. Immediate treatment with combination antiretroviral therapy Antiretroviral therapy Antiretroviral therapy (ART) targets the replication cycle of the human immunodeficiency virus (HIV) and is classified based on the viral enzyme or mechanism that is inhibited. The goal of therapy is to suppress viral replication to reach the outcome of undetected viral load. Anti-HIV Drugs is recommended.
Last updated: Jun 24, 2025
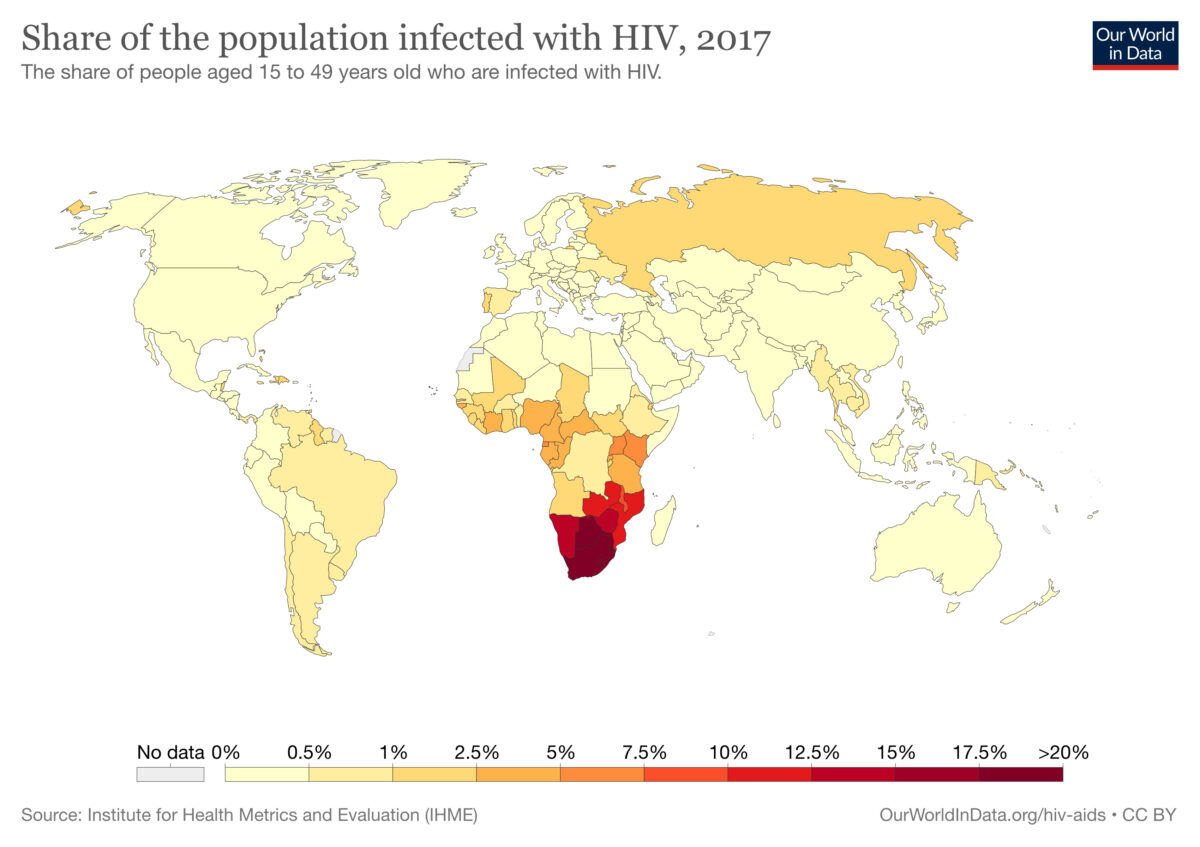
2017 world map of HIV infections (in people aged 15 to 49 years of age):
The colors indicate the percent of population with human immunodeficiency virus (HIV) infection in each country. The information regarding the corresponding percent of population (designated color) is below the world map.
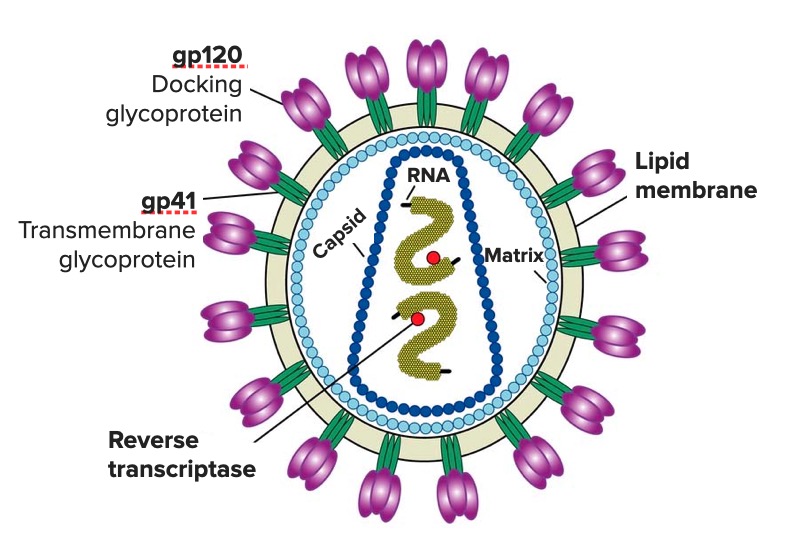
Diagram of the HIV virion featuring the structure of its genome and its main enzymes and glycoproteins (gp120 and gp41)
Image: “Diagram of the HIV virus” by US National Institute of Health. License: Public DomainSexual:
Parenteral:
Vertical:
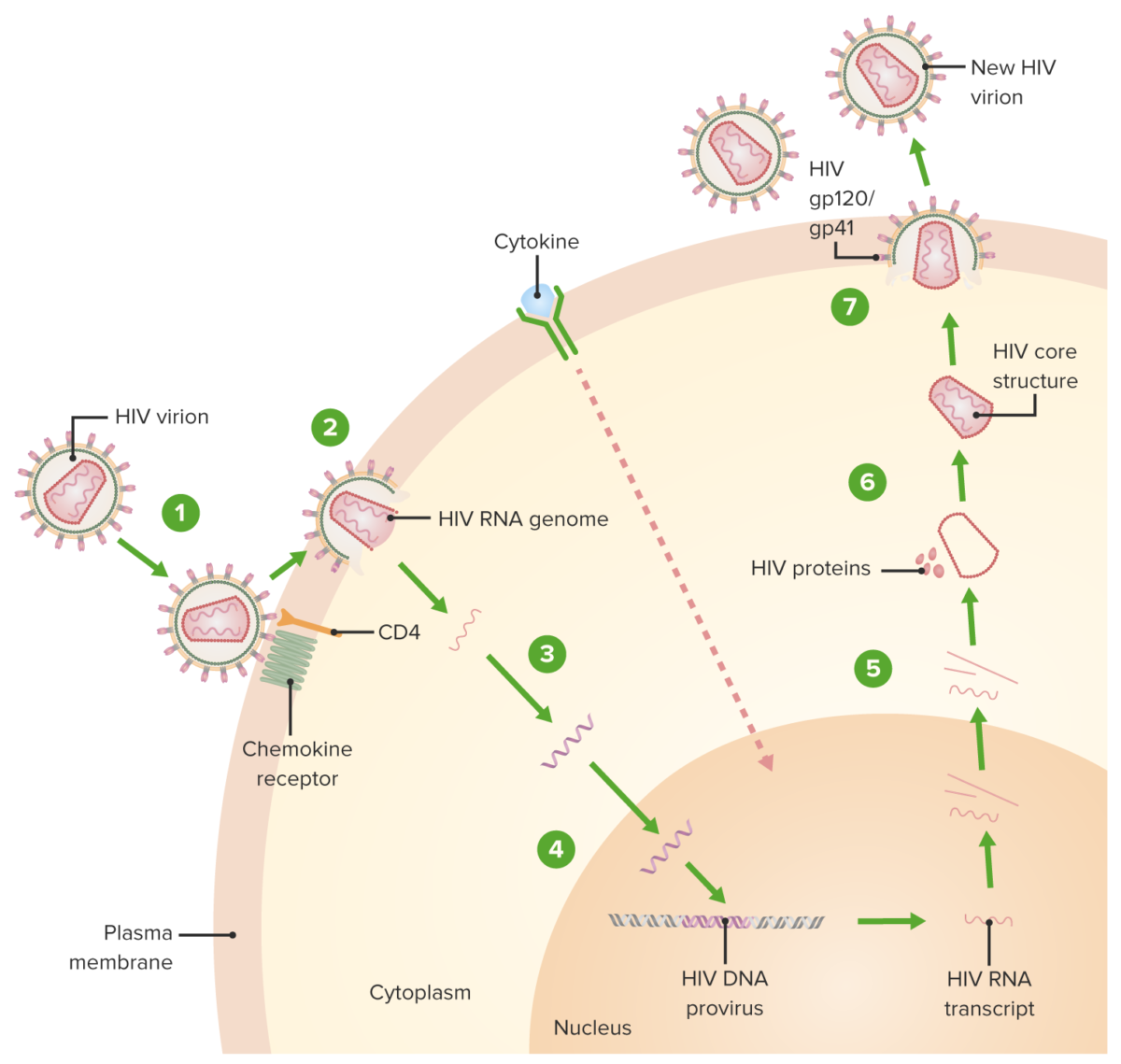
HIV replication cycle:
1. Virion binds the CD4 receptor and a chemokine receptor, followed by a conformational change that facilitates fusion of the virion and the host cell.
2. A capsid protein shell (surrounding the viral RNA and proteins) is uncoated as the virion traverses the cytoplasm.
3. Reverse transcriptase-mediated synthesis of proviral DNA occurs.
4. Viral DNA is transported across the nucleus and integrated into the host DNA, facilitated by integrase.
5. Viral DNA is transcribed, and multiple copies of new HIV RNA form and are transported to the cytoplasm. New HIV RNA becomes the genome of a new virus. Cytokine activation of the cell also occurs.
6. New viral RNA + proteins + enzymes move to the cell surface and form a noninfectious particle.
7. Particle (viral RNA + proteins) eventually buds out of the host cell with the immature HIV. Viral protein protease (enzyme) then cleaves newly synthesized polyproteins producing a mature HIV.
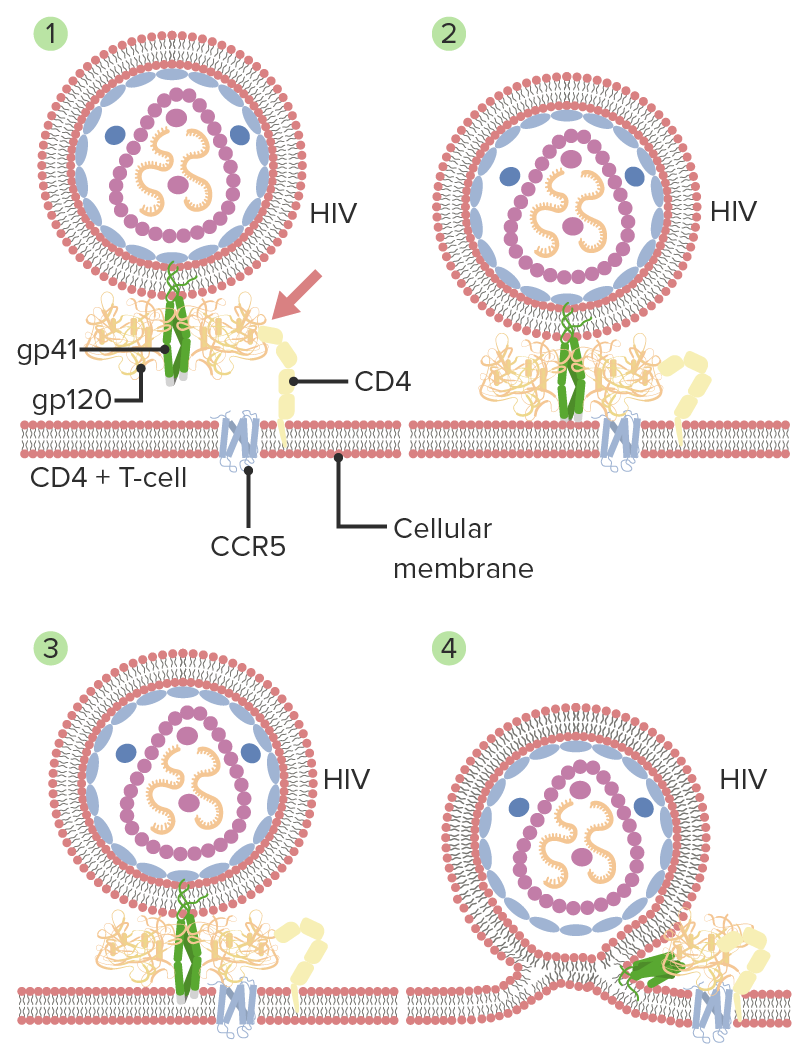
Mechanism of HIV entry and membrane fusion:
1. Gp120 HIV interacts with CD4 (host cell).
2. A secondary interaction with another receptor CCR5 follows, with a conformational change in gp120.
3. The tips of gp41 are inserted into the cellular membrane.
4. Gp41 folds in half and forms coiled coils. The viral and cellular membranes pull together, leading to fusion.
Acute phase Acute phase Short Bowel Syndrome (infection, dissemination, retroviral syndrome):
Chronic phase/clinical latency:
AIDS:
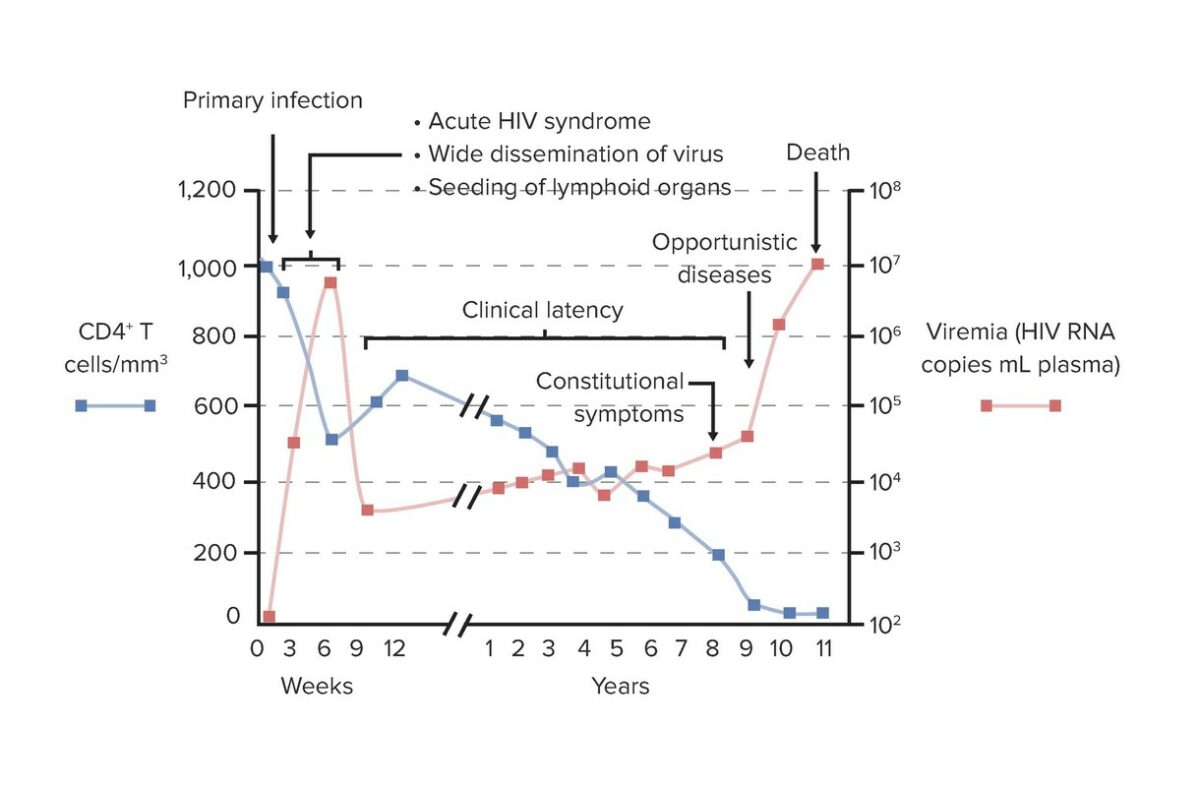
Graph of the relationship between CD4+ T cell count and viral load during the clinical course of HIV infection and AIDS:
In primary/acute infection (initial infection, dissemination, and acute retroviral syndrome), an increase in viral load (viremia) with declining CD4+ T cells is noted. This period lasts weeks. In the period of clinical latency, low-level but sustained viral replication occurs and gradual decline of CD4+ cells is seen. Progression to AIDS is the result of the breakdown of host defenses, with depleted CD4+ T cells and increasing viral load. This process predisposes to opportunistic infections.
The Centers for Disease Control and Prevention (CDC) classification notes that CD4+ T lymphocyte count Lymphocyte count The number of lymphocytes per unit volume of blood. Lymphocytosis is:
| CD 4+ T cell categories/stage | 1 | 2 | 3 |
|---|---|---|---|
| Clinical categories | CD4+ ≥ 500 cells/µL | CD4+ 200–499 cells/µL | CD4+ < 200 cells/µL* |
| A: Asymptomatic, acute HIV HIV Anti-HIV Drugs, persistent lymphadenopathy Lymphadenopathy Lymphadenopathy is lymph node enlargement (> 1 cm) and is benign and self-limited in most patients. Etiologies include malignancy, infection, and autoimmune disorders, as well as iatrogenic causes such as the use of certain medications. Generalized lymphadenopathy often indicates underlying systemic disease. Lymphadenopathy | A1 | A2 | A3 |
| B: Symptomatic, not A or C | B1 | B2 | B3 |
| C: AIDS, including opportunistic infections Infections Invasion of the host organism by microorganisms or their toxins or by parasites that can cause pathological conditions or diseases. Chronic Granulomatous Disease, neurologic disease, and tumors | C1 | C2 | C3 |
Acute retroviral syndrome ( acute phase Acute phase Short Bowel Syndrome):
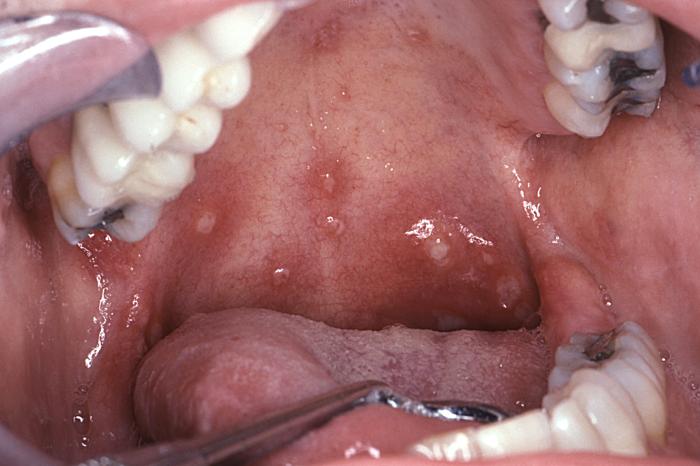
Aphthous stomatitis typical of that seen in acute retroviral syndrome
Image: “6055” by CDC/ Sol Silverman, Jr., DDS. License: Public Domain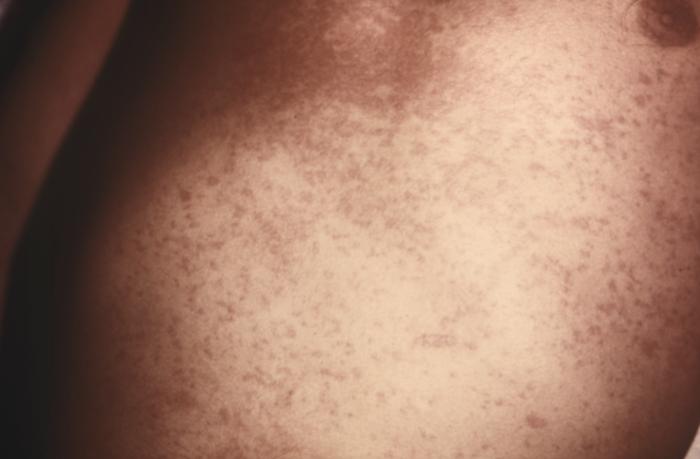
Viral maculopapular exanthem seen in acute retroviral syndrome
Image: “14925” by CDC/ Dr. Gavin Hart. License: Public DomainChronic infection (clinical latency):
AIDS:
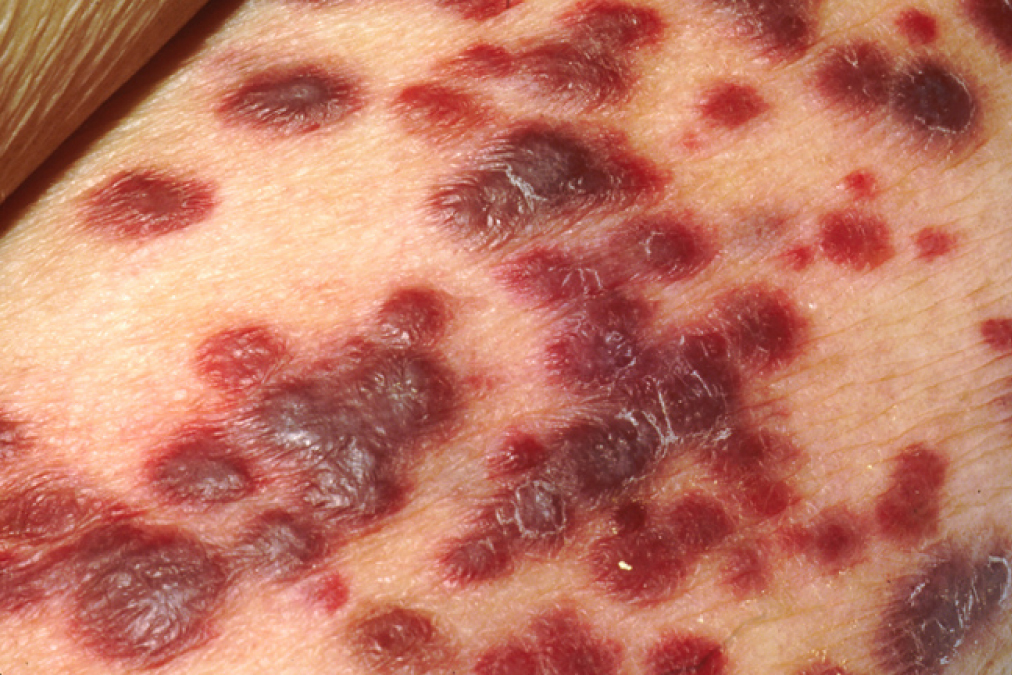
Cutaneous lesions of Kaposi’s sarcoma
Image: “Kaposi’s sarcoma” by OpenStax College. License: CC BY 3.0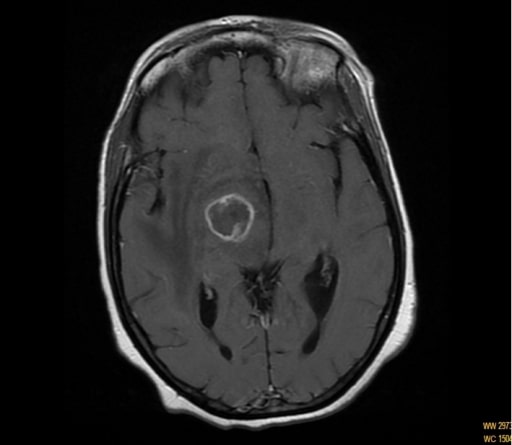
Toxoplasmosis and AIDS:
Magnetic resonance imaging (MRI) showing ring enhancing of the capsule-thalamic lesion in a patient with hemichorea-hemiballismus
Cryptococcosis:
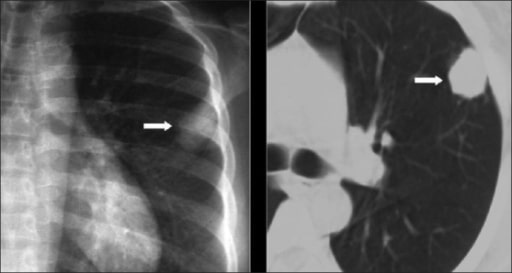
Chest X-ray and computed tomography (CT) show a solitary pulmonary nodule. The diagnosis of cryptococcosis was confirmed on biopsy.

Mycobacterium avium complex: A man with HIV evaluated for severe right-sided upper abdominal pain. Despite treatment, he developed an enlarged supraclavicular lymph node abscess. Aspirate revealed MAC.
Image: “Left supraclavicular abscessed lymph node” by Department of Internal Medicine, Division of Infectious Diseases, University of Michigan Health System, Ann Arbor, Michigan, USA. License: CC BY 2.0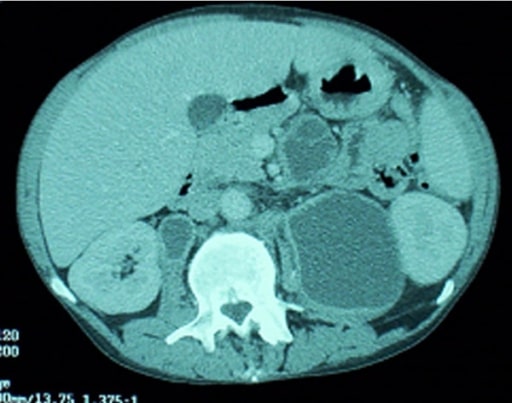
Mycobacterium avium complex:
A man with HIV evaluated for severe right-sided upper abdominal pain. An abdominal CT revealed multiple enlarged necrotic intra-abdominal lymph nodes.
Pneumocystis pneumonia:
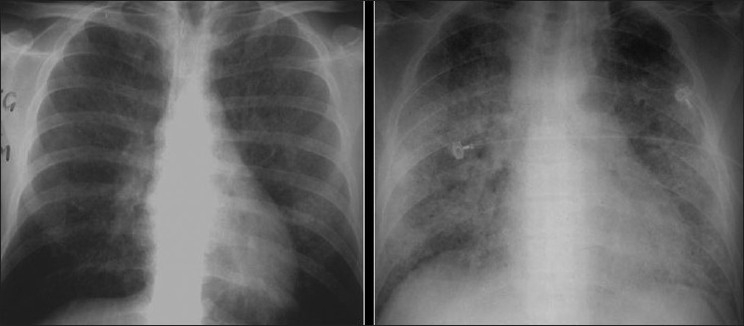
2 chest radiographs show ground-glass appearance. The right chest X-ray findings mimic pulmonary edema.
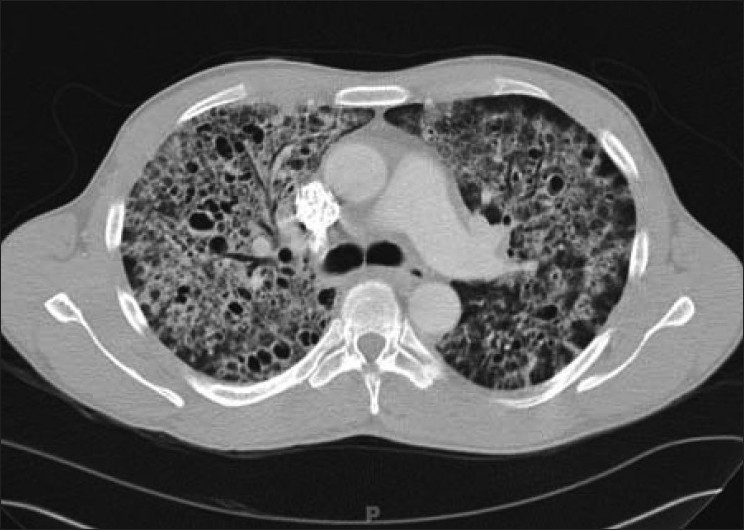
Chest CT showing the hallmark of Pneumocystis pneumonia in a clinical setting of immune compromise:
Note the ground-glass attenuation with a geographic or mosaic distribution.
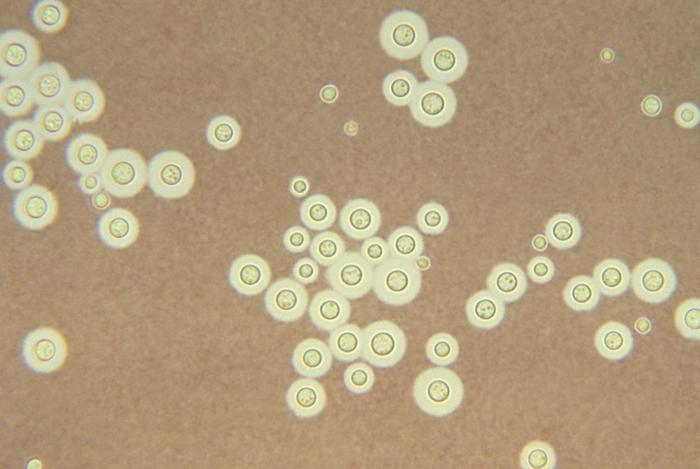
India Ink mount of cryptococcus:
Note the capsule surrounding the budding yeast.
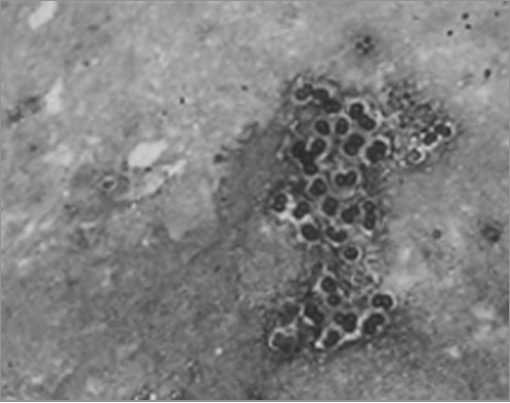
P. jiroveci:
Methenamine silver stain demonstrating clusters of P. jiroveci cysts in the sputum
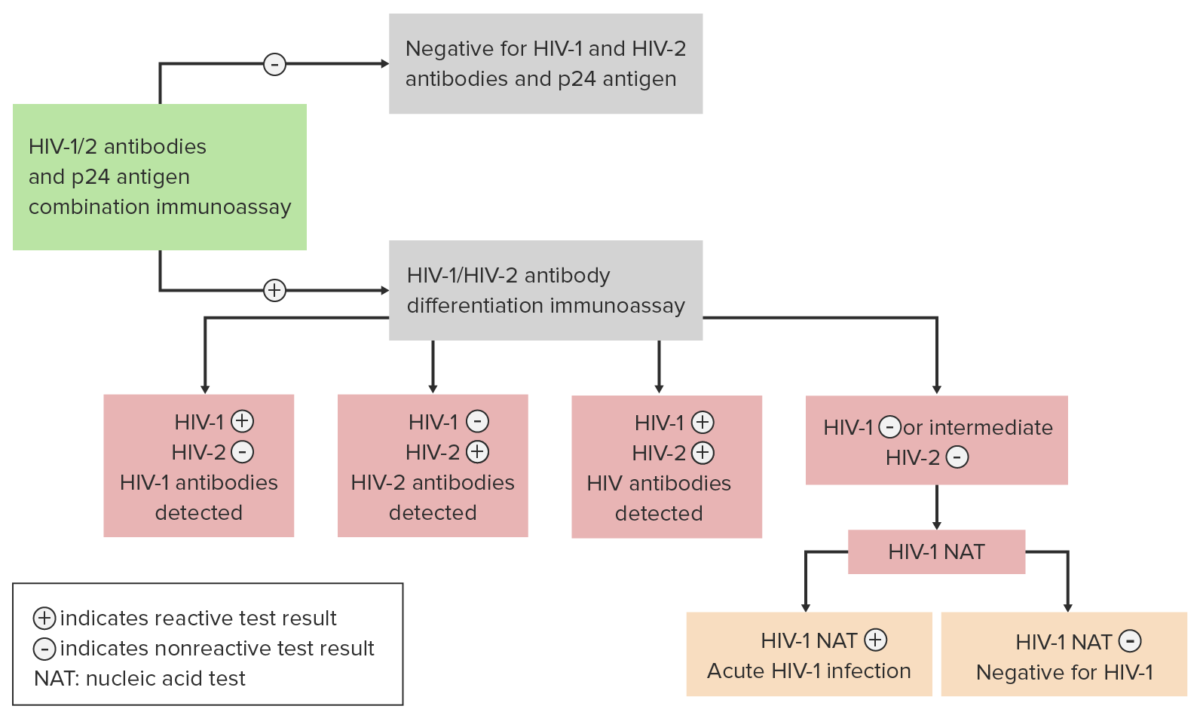
Algorithm for the diagnosis and differentiation between an HIV infection produced by subtype HIV-1 and subtype HIV-2
Image by Lecturio.| Stage | < 1 year | 1–5 years | ≥ 6 years |
|---|---|---|---|
| 0 | NA | NA | NA |
| 1 | ≥ 1,500 cells/µL | ≥ 1,000 cells/µL | ≥ 500 cells/µL |
| 2 | 750–1,499 cells/µL | 500–999 cells/µL | 200–499 cells/µL |
| 3 (AIDS) | < 750 cells/µL | < 500 cells/µL | < 200 cells/µL |