Subarachnoid hemorrhage (SAH) is a type of cerebrovascular accident Cerebrovascular accident An ischemic stroke (also known as cerebrovascular accident) is an acute neurologic injury that occurs as a result of brain ischemia; this condition may be due to cerebral blood vessel occlusion by thrombosis or embolism, or rarely due to systemic hypoperfusion. Ischemic Stroke (stroke) resulting from intracranial hemorrhage into the subarachnoid space between the arachnoid and the pia mater Pia mater The innermost layer of the three meninges covering the brain and spinal cord. It is the fine vascular membrane that lies under the arachnoid and the dura mater. Meninges: Anatomy layers of the meninges Meninges The brain and the spinal cord are enveloped by 3 overlapping layers of connective tissue called the meninges. The layers are, from the most external layer to the most internal layer, the dura mater, arachnoid mater, and pia mater. Between these layers are 3 potential spaces called the epidural, subdural, and subarachnoid spaces. Meninges: Anatomy surrounding the brain Brain The part of central nervous system that is contained within the skull (cranium). Arising from the neural tube, the embryonic brain is comprised of three major parts including prosencephalon (the forebrain); mesencephalon (the midbrain); and rhombencephalon (the hindbrain). The developed brain consists of cerebrum; cerebellum; and other structures in the brain stem. Nervous System: Anatomy, Structure, and Classification. Most SAHs originate from a saccular aneurysm Aneurysm An aneurysm is a bulging, weakened area of a blood vessel that causes an abnormal widening of its diameter > 1.5 times the size of the native vessel. Aneurysms occur more often in arteries than in veins and are at risk of dissection and rupture, which can be life-threatening. Thoracic Aortic Aneurysms in the circle of Willis but may also occur as a result of trauma, uncontrolled hypertension Uncontrolled hypertension Although hypertension is defined as a blood pressure of > 130/80 mm Hg, individuals can present with comorbidities of severe asymptomatic or "uncontrolled" hypertension (≥ 180 mm Hg systolic and/or ≥ 120 mm Hg diastolic) that carries with it a significant risk of morbidity and mortality. Uncontrolled Hypertension, vasculitis Vasculitis Inflammation of any one of the blood vessels, including the arteries; veins; and rest of the vasculature system in the body. Systemic Lupus Erythematosus, anticoagulant use, or stimulant use. The most classic symptom is a sudden-onset (thunderclap) headache Headache The symptom of pain in the cranial region. It may be an isolated benign occurrence or manifestation of a wide variety of headache disorders. Brain Abscess along with neck stiffness Neck Stiffness Meningitis, vomiting Vomiting The forcible expulsion of the contents of the stomach through the mouth. Hypokalemia, a decreased level of consciousness, and seizure. As with any stroke, focal neurologic deficits Neurologic Deficits High-Risk Headaches are commonly present, and rapid neurologic deterioration may ensue without prompt diagnosis and intervention. An SAH should be suspected in any person presenting with thunderclap headache Headache The symptom of pain in the cranial region. It may be an isolated benign occurrence or manifestation of a wide variety of headache disorders. Brain Abscess and neurologic symptoms, and the diagnosis can be confirmed with neuroimaging Neuroimaging Non-invasive methods of visualizing the central nervous system, especially the brain, by various imaging modalities. Febrile Infant or lumbar puncture Lumbar Puncture Febrile Infant (LP). Treatment consists of reversal of anticoagulation Anticoagulation Pulmonary Hypertension Drugs, control of blood pressure, and neurosurgical intervention to contain the bleed and/or relieve elevated intracranial pressure Intracranial Pressure Idiopathic Intracranial Hypertension ( ICP ICP Normal intracranial pressure (ICP) is defined as < 15 mm Hg, whereas pathologically increased ICP is any pressure ≥ 20 mm Hg. Increased ICP may result from several etiologies, including trauma, intracranial hemorrhage, mass lesions, cerebral edema, increased CSF production, and decreased CSF absorption. Increased Intracranial Pressure (ICP)). Even with prompt neurosurgical intervention, SAH carries a high mortality Mortality All deaths reported in a given population. Measures of Health Status rate.
Last updated: Jul 25, 2023
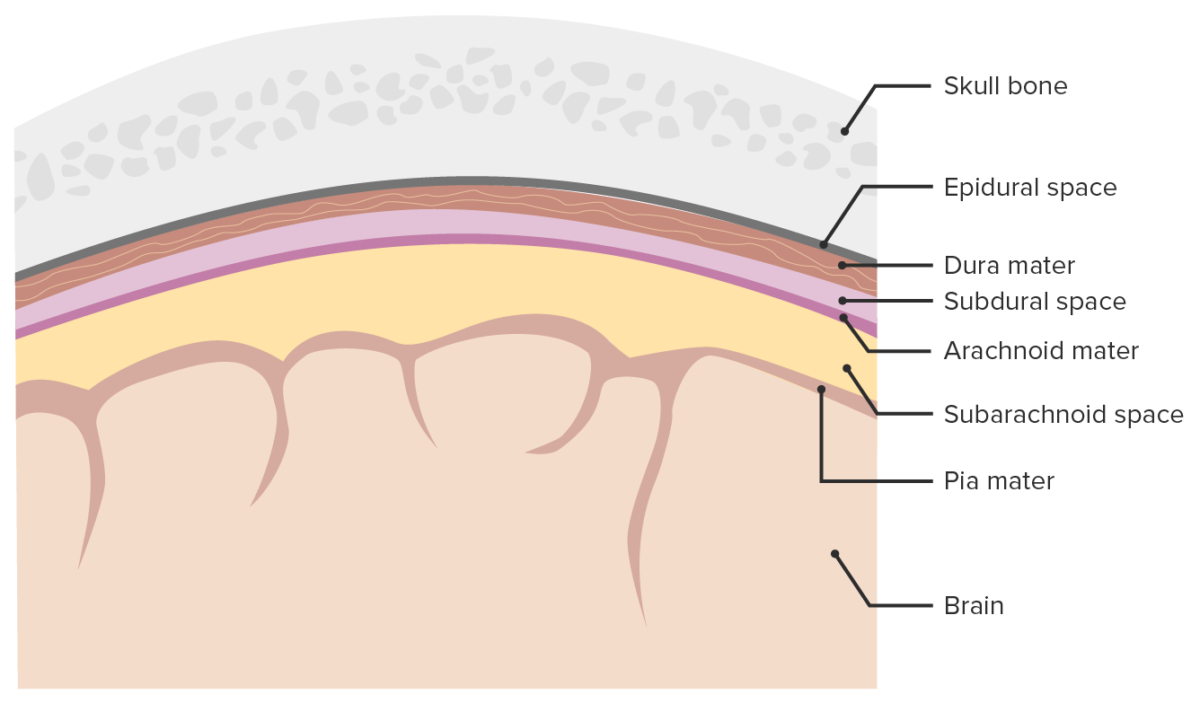
Meninges and meningeal spaces:
The image depicts the 3 layers (dura mater, arachnoid mater, pia mater) surrounding the brain and spinal cord. The meninges serve as mechanical protection of the CNS. They also support the cerebral and spinal blood vessels and allow for passage of the CSF. The subarachnoid space is filled with CSF.
Given that saccular aneurysm Aneurysm An aneurysm is a bulging, weakened area of a blood vessel that causes an abnormal widening of its diameter > 1.5 times the size of the native vessel. Aneurysms occur more often in arteries than in veins and are at risk of dissection and rupture, which can be life-threatening. Thoracic Aortic Aneurysms is the most common etiology of SAH, this section will focus on the pathogenesis of saccular aneurysm Aneurysm An aneurysm is a bulging, weakened area of a blood vessel that causes an abnormal widening of its diameter > 1.5 times the size of the native vessel. Aneurysms occur more often in arteries than in veins and are at risk of dissection and rupture, which can be life-threatening. Thoracic Aortic Aneurysms rupture. Events that occur after the rupture itself are common to other etiologies of SAH.
The classic presenting symptom of SAH is a thunderclap headache Headache The symptom of pain in the cranial region. It may be an isolated benign occurrence or manifestation of a wide variety of headache disorders. Brain Abscess. There are other presenting symptoms as well.
Any thunderclap headache Headache The symptom of pain in the cranial region. It may be an isolated benign occurrence or manifestation of a wide variety of headache disorders. Brain Abscess presenting with or without neurologic symptoms/signs or altered mental status Altered Mental Status Sepsis in Children should be emergently evaluated with neuroimaging Neuroimaging Non-invasive methods of visualizing the central nervous system, especially the brain, by various imaging modalities. Febrile Infant. Noncontrast CT is readily available at most acute care hospitals and is the initial test of choice.
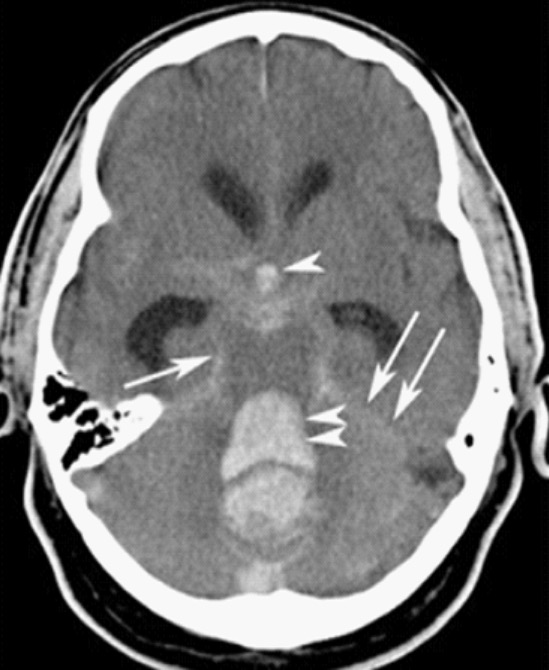
Subarachnoid hemorrhage (SAH):
CT scan showing intracranial bleeding
An LP should be performed promptly (despite negative CT if clinical suspicion of SAH is high). Studies should include:
Several scales Scales Dry or greasy masses of keratin that represent thickened stratum corneum. Secondary Skin Lesions are utilized clinically in the diagnosis and grading Grading Methods which attempt to express in replicable terms the level of cell differentiation in neoplasms as increasing anaplasia correlates with the aggressiveness of the neoplasm. Grading, Staging, and Metastasis of severity in SAH. The Hunt and Hess grading Grading Methods which attempt to express in replicable terms the level of cell differentiation in neoplasms as increasing anaplasia correlates with the aggressiveness of the neoplasm. Grading, Staging, and Metastasis system is among the most commonly used in clinical medicine.
| Grade | Neurologic findings | Mortality Mortality All deaths reported in a given population. Measures of Health Status rate (5) |
|---|---|---|
| 1 | Asymptomatic or mild headache Headache The symptom of pain in the cranial region. It may be an isolated benign occurrence or manifestation of a wide variety of headache disorders. Brain Abscess and slight nuchal rigidity Nuchal Rigidity Meningitis | 1 |
| 2 | Severe headache Headache The symptom of pain in the cranial region. It may be an isolated benign occurrence or manifestation of a wide variety of headache disorders. Brain Abscess, stiff neck Neck The part of a human or animal body connecting the head to the rest of the body. Peritonsillar Abscess, no neurologic deficit except cranial nerve (CN) palsy Palsy paralysis of an area of the body, thus incapable of voluntary movement Cranial Nerve Palsies | 5 |
| 3 | Drowsy or confused, mild focal neurologic deficit Focal Neurologic Deficit Intracerebral Hemorrhage | 19 |
| 4 | Stuporous, moderate or severe hemiparesis Hemiparesis The term hemiparesis refers to mild to moderate weakness involving one side of the body. Epidural Hemorrhage | 42 |
| 5 | Coma Coma Coma is defined as a deep state of unarousable unresponsiveness, characterized by a score of 3 points on the GCS. A comatose state can be caused by a multitude of conditions, making the precise epidemiology and prognosis of coma difficult to determine. Coma, decerebrate posturing Decerebrate posturing A condition characterized by abnormal posturing of the limbs that is associated with injury to the brainstem. This may occur as a clinical manifestation or induced experimentally in animals. The extensor reflexes are exaggerated leading to rigid extension of the limbs accompanied by hyperreflexia and opisthotonus. This condition is usually caused by lesions which occur in the region of the brainstem that lies between the red nuclei and the vestibular nuclei. In contrast, decorticate rigidity is characterized by flexion of the elbows and wrists with extension of the legs and feet. The causative lesion for this condition is located above the red nuclei and usually consists of diffuse cerebral damage. Increased Intracranial Pressure (ICP) | 77 |
Consult neurosurgery Neurosurgery Neurosurgery is a specialized field focused on the surgical management of pathologies of the brain, spine, spinal cord, and peripheral nerves. General neurosurgery includes cases of trauma and emergencies. There are a number of specialized neurosurgical practices, including oncologic neurosurgery, spinal neurosurgery, and pediatric neurosurgery. Neurosurgery and/or endovascular interventionist! The goal is to stop the bleeding, prevent rebleeding, manage ICP ICP Normal intracranial pressure (ICP) is defined as < 15 mm Hg, whereas pathologically increased ICP is any pressure ≥ 20 mm Hg. Increased ICP may result from several etiologies, including trauma, intracranial hemorrhage, mass lesions, cerebral edema, increased CSF production, and decreased CSF absorption. Increased Intracranial Pressure (ICP) to prevent secondary ischemia Ischemia A hypoperfusion of the blood through an organ or tissue caused by a pathologic constriction or obstruction of its blood vessels, or an absence of blood circulation. Ischemic Cell Damage. Possible interventions include:
Monitoring should be performed in an ICU ICU Hospital units providing continuous surveillance and care to acutely ill patients. West Nile Virus by specially trained staff equipped to continuously and simultaneously address the following:
It is reasonable to offer screening Screening Preoperative Care ( neuroimaging Neuroimaging Non-invasive methods of visualizing the central nervous system, especially the brain, by various imaging modalities. Febrile Infant) to 1st-degree relatives of patients Patients Individuals participating in the health care system for the purpose of receiving therapeutic, diagnostic, or preventive procedures. Clinician–Patient Relationship with SAH for saccular aneurysms Saccular aneurysms Brain Aneurysms.