Inflammation is a complex set of responses to infection and injury involving leukocytes Leukocytes White blood cells. These include granular leukocytes (basophils; eosinophils; and neutrophils) as well as non-granular leukocytes (lymphocytes and monocytes). White Myeloid Cells: Histology as the principal cellular mediators in the body’s defense against pathogenic organisms. Inflammation is also seen as a response to tissue injury in the process of wound healing Wound healing Wound healing is a physiological process involving tissue repair in response to injury. It involves a complex interaction of various cell types, cytokines, and inflammatory mediators. Wound healing stages include hemostasis, inflammation, granulation, and remodeling. Wound Healing. The 5 cardinal signs of inflammation are pain Pain An unpleasant sensation induced by noxious stimuli which are detected by nerve endings of nociceptive neurons. Pain: Types and Pathways, heat, redness, swelling, and loss of function. The major cellular response involves neutrophils Neutrophils Granular leukocytes having a nucleus with three to five lobes connected by slender threads of chromatin, and cytoplasm containing fine inconspicuous granules and stainable by neutral dyes. Innate Immunity: Phagocytes and Antigen Presentation and macrophages Macrophages The relatively long-lived phagocytic cell of mammalian tissues that are derived from blood monocytes. Main types are peritoneal macrophages; alveolar macrophages; histiocytes; kupffer cells of the liver; and osteoclasts. They may further differentiate within chronic inflammatory lesions to epithelioid cells or may fuse to form foreign body giant cells or langhans giant cells. Innate Immunity: Phagocytes and Antigen Presentation to phagocytose and lyse the injurious organism or repair necrosed tissue after injury. Inflammation can be pathologic if it is prolonged or when normal processes create an excessive response (such as with atherosclerosis Atherosclerosis Atherosclerosis is a common form of arterial disease in which lipid deposition forms a plaque in the blood vessel walls. Atherosclerosis is an incurable disease, for which there are clearly defined risk factors that often can be reduced through a change in lifestyle and behavior of the patient. Atherosclerosis). There are multiple mediators of inflammation that overlap with innate immunity Innate immunity The capacity of a normal organism to remain unaffected by microorganisms and their toxins. It results from the presence of naturally occurring anti-infective agents, constitutional factors such as body temperature and immediate acting immune cells such as natural killer cells. Innate Immunity: Phagocytes and Antigen Presentation when they respond to injurious stimuli Injurious Stimuli Cell Injury and Death. Inflammation can become chronic, resulting in the formation of granulomas Granulomas A relatively small nodular inflammatory lesion containing grouped mononuclear phagocytes, caused by infectious and noninfectious agents. Sarcoidosis, tissue damage, and the loss of organ function.
Last updated: May 17, 2024
Inflammation is a complex set of responses to infection and injury, involving leukocytes Leukocytes White blood cells. These include granular leukocytes (basophils; eosinophils; and neutrophils) as well as non-granular leukocytes (lymphocytes and monocytes). White Myeloid Cells: Histology as the principal cellular mediators in the body’s defense against pathogenic organisms. Inflammation is also seen as a response to tissue injury in the process of wound healing Wound healing Wound healing is a physiological process involving tissue repair in response to injury. It involves a complex interaction of various cell types, cytokines, and inflammatory mediators. Wound healing stages include hemostasis, inflammation, granulation, and remodeling. Wound Healing.
Types:
Triggers of acute inflammation:
Characteristics of acute inflammation:
Characteristics of chronic inflammation:
Innate inflammation:
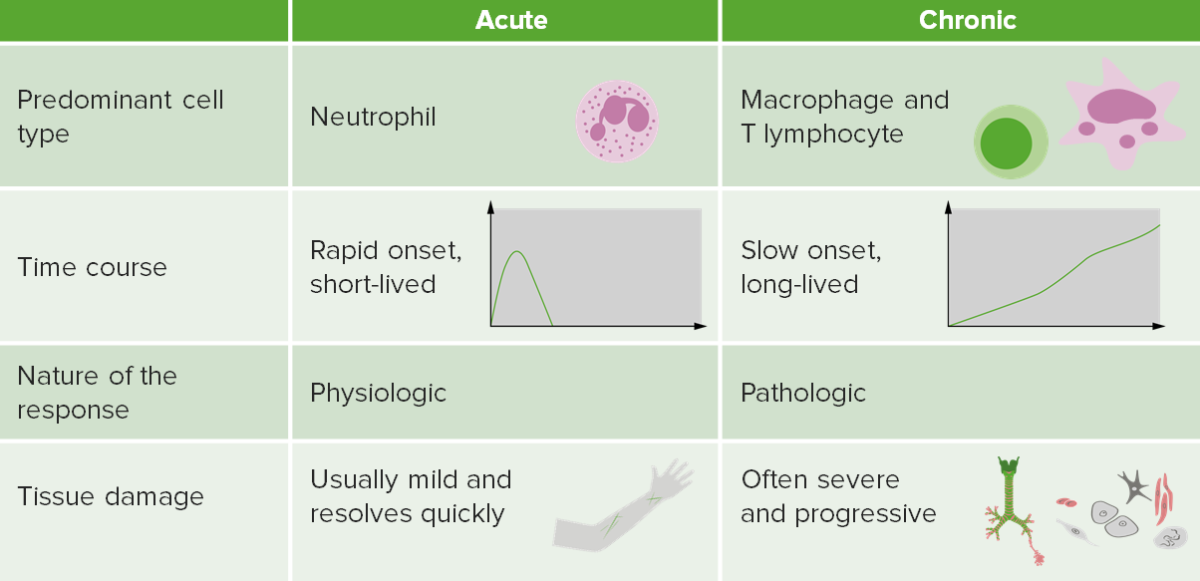
Contrasting acute and chronic inflammation
Image by Lecturio.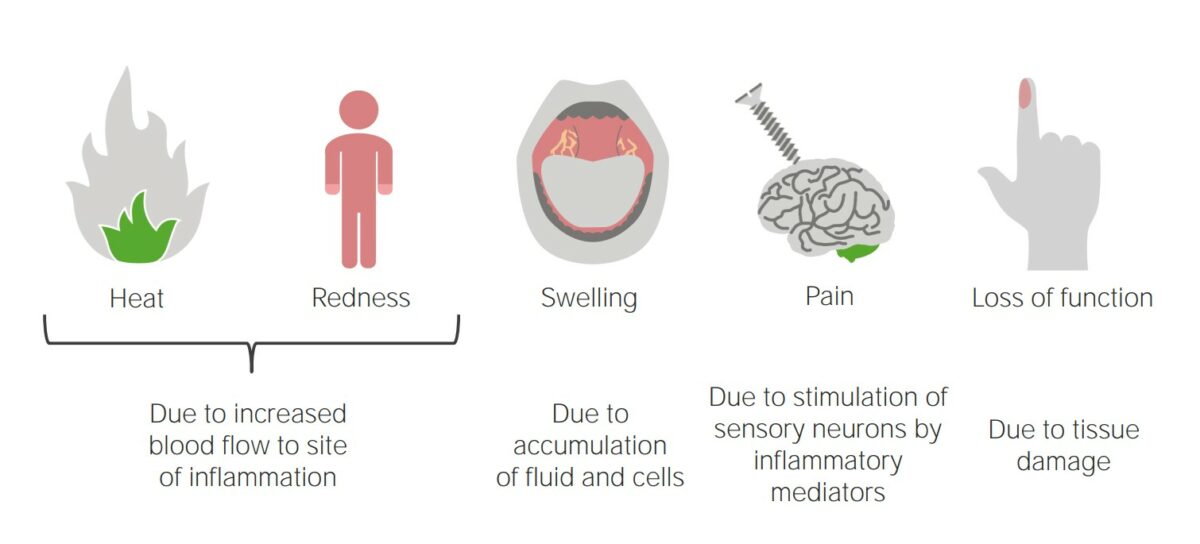
Acute inflammatory response
Image by Lecturio.There are multiple mediators of inflammation that bring inflammatory cells into the sites of injury and overlap with innate immunity Innate immunity The capacity of a normal organism to remain unaffected by microorganisms and their toxins. It results from the presence of naturally occurring anti-infective agents, constitutional factors such as body temperature and immediate acting immune cells such as natural killer cells. Innate Immunity: Phagocytes and Antigen Presentation when they respond to injurious stimuli Injurious Stimuli Cell Injury and Death.
| Reactant | Function |
|---|---|
| CRP |
|
| ESR ESR Soft Tissue Abscess |
|
| Haptoglobin |
|
| SAA | Causes immune cell recruitment Recruitment Skeletal Muscle Contraction at the site of inflammation |
Vasodilation Vasodilation The physiological widening of blood vessels by relaxing the underlying vascular smooth muscle. Pulmonary Hypertension Drugs:
Increased permeability:
Cellular release of chemicals causes:
Neutrophil recruitment Recruitment Skeletal Muscle Contraction:
Phagocytosis Phagocytosis The engulfing and degradation of microorganisms; other cells that are dead, dying, or pathogenic; and foreign particles by phagocytic cells (phagocytes). Innate Immunity: Phagocytes and Antigen Presentation and killing:
Mediator release:
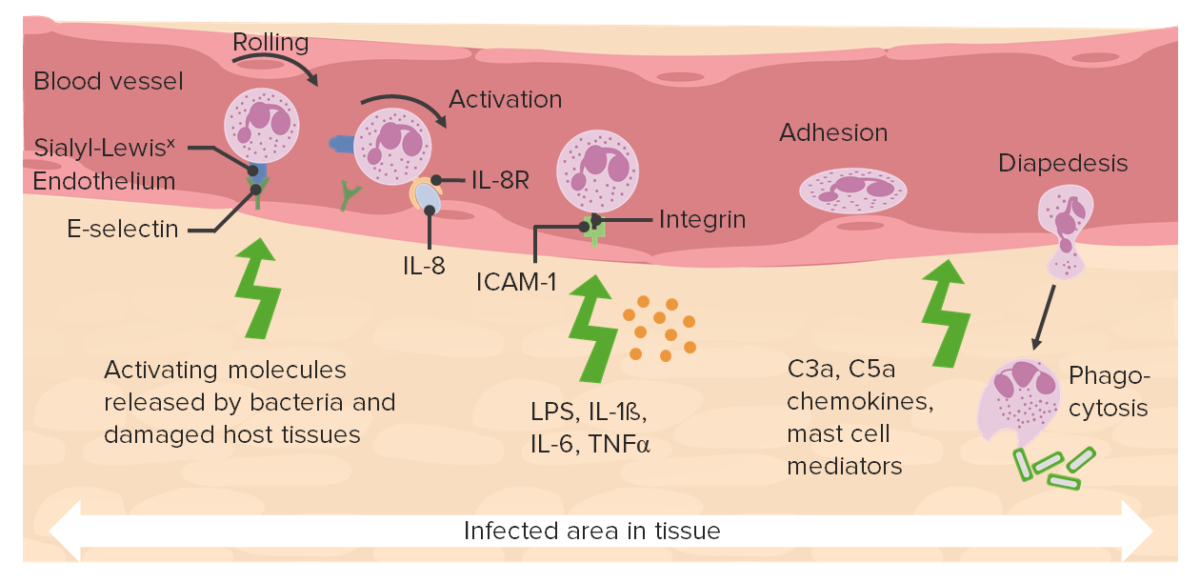
Interplay of various chemokines:
Various ligands and receptors bind and promote neutrophil extravasation.
C3a and C5a: complement
ICAM-1: intercellular adhesion molecule 1
LPS: lipopolysaccharides
TNF-α: tumor necrosis factor alpha
Inflammation can resolve, form abscesses, or become chronic.
Acute inflammation with persistent reinjury can lead to chronic inflammation. Chronic inflammation can also be seen with viral infections Infections Invasion of the host organism by microorganisms or their toxins or by parasites that can cause pathological conditions or diseases. Chronic Granulomatous Disease and autoimmune disorders.
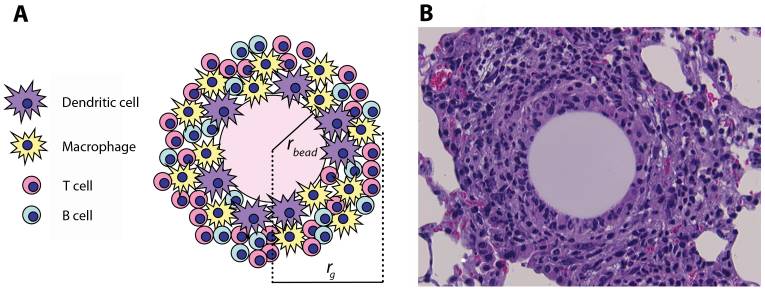
(A) Schematic representation and (B) histological appearance of an artificial pulmonary granuloma induced in mouse 4 days after injection of purified protein-derivative (PPD)-coated beads (H&E staining; magnification: ×800)
rbead: radius of the bead
rg: radius of the granuloma
Examples of inflammation becoming a pathologic response:
Disorders due to deficiency in some mediators of the inflammatory response: