Eicosanoids are cell-signaling molecules produced from arachidonic acid Arachidonic Acid An unsaturated, essential fatty acid. It is found in animal and human fat as well as in the liver, brain, and glandular organs, and is a constituent of animal phosphatides. It is formed by the synthesis from dietary linoleic acid and is a precursor in the biosynthesis of prostaglandins, thromboxanes, and leukotrienes. Nonsteroidal Antiinflammatory Drugs (NSAIDs). With the action of phospholipase A2 Phospholipase A2 Phospholipases that hydrolyze the Acyl group attached to the 2-position of phosphoglycerides. Nephrotic Syndrome, arachidonic acid Arachidonic Acid An unsaturated, essential fatty acid. It is found in animal and human fat as well as in the liver, brain, and glandular organs, and is a constituent of animal phosphatides. It is formed by the synthesis from dietary linoleic acid and is a precursor in the biosynthesis of prostaglandins, thromboxanes, and leukotrienes. Nonsteroidal Antiinflammatory Drugs (NSAIDs) is released from the plasma membrane Plasma membrane A cell membrane (also known as the plasma membrane or plasmalemma) is a biological membrane that separates the cell contents from the outside environment. A cell membrane is composed of a phospholipid bilayer and proteins that function to protect cellular DNA and mediate the exchange of ions and molecules. The Cell: Cell Membrane. The different families of eicosanoids, which are prostaglandins (PGs), thromboxanes (TXA2s), prostacyclin ( PGI PGI An aldose-ketose isomerase that catalyzes the reversible interconversion of glucose 6-phosphate and fructose 6-phosphate. In prokaryotic and eukaryotic organisms it plays an essential role in glycolytic and gluconeogenic pathways. In mammalian systems the enzyme is found in the cytoplasm and as a secreted protein. This secreted form of glucose-6-phosphate isomerase has been referred to as autocrine motility factor or neuroleukin, and acts as a cytokine which binds to the autocrine motility factor receptor. Deficiency of the enzyme in humans is an autosomal recessive trait, which results in congenital nonspherocytic hemolytic anemia. Glycolysis2), lipoxins (LXs), and leukotrienes (LTs), emerge from a series of reactions catalyzed by different enzymes Enzymes Enzymes are complex protein biocatalysts that accelerate chemical reactions without being consumed by them. Due to the body's constant metabolic needs, the absence of enzymes would make life unsustainable, as reactions would occur too slowly without these molecules. Basics of Enzymes. The LTs and LXs are products of the lipoxygenase (LOX) pathway. The remaining eicosanoids are produced from the cyclooxygenase Cyclooxygenase Nonsteroidal Antiinflammatory Drugs (NSAIDs) (COX) pathway, which involves 2 enzymes Enzymes Enzymes are complex protein biocatalysts that accelerate chemical reactions without being consumed by them. Due to the body's constant metabolic needs, the absence of enzymes would make life unsustainable, as reactions would occur too slowly without these molecules. Basics of Enzymes, COX-1 and COX-2. Eicosanoids are involved in various physiological and pathological processes. Thromboxanes cause platelet aggregation Platelet aggregation The attachment of platelets to one another. This clumping together can be induced by a number of agents (e.g., thrombin; collagen) and is part of the mechanism leading to the formation of a thrombus. Hemostasis and are potent vasoconstrictors. Leukotrienes mediate allergic responses, while LXs have anti-inflammatory activities. Principal actions of PGs include vasodilation Vasodilation The physiological widening of blood vessels by relaxing the underlying vascular smooth muscle. Pulmonary Hypertension Drugs, smooth muscle contraction Smooth muscle contraction Smooth muscle is primarily found in the walls of hollow structures and some visceral organs, including the walls of the vasculature, GI, respiratory, and genitourinary tracts. Smooth muscle contracts more slowly and is regulated differently than skeletal muscle. Smooth muscle can be stimulated by nerve impulses, hormones, metabolic factors (like pH, CO2 or O2 levels), its own intrinsic pacemaker ability, or even mechanical stretch. Smooth Muscle Contraction, and inflammation Inflammation Inflammation is a complex set of responses to infection and injury involving leukocytes as the principal cellular mediators in the body's defense against pathogenic organisms. Inflammation is also seen as a response to tissue injury in the process of wound healing. The 5 cardinal signs of inflammation are pain, heat, redness, swelling, and loss of function. Inflammation. Prostacyclin, a member of the PG family, has a potent vasodilatory effect. Both biologic actions and inhibitions of eicosanoids are mechanisms used in pharmacologic agents for various medical conditions and desired clinical effects.
Last updated: May 17, 2024
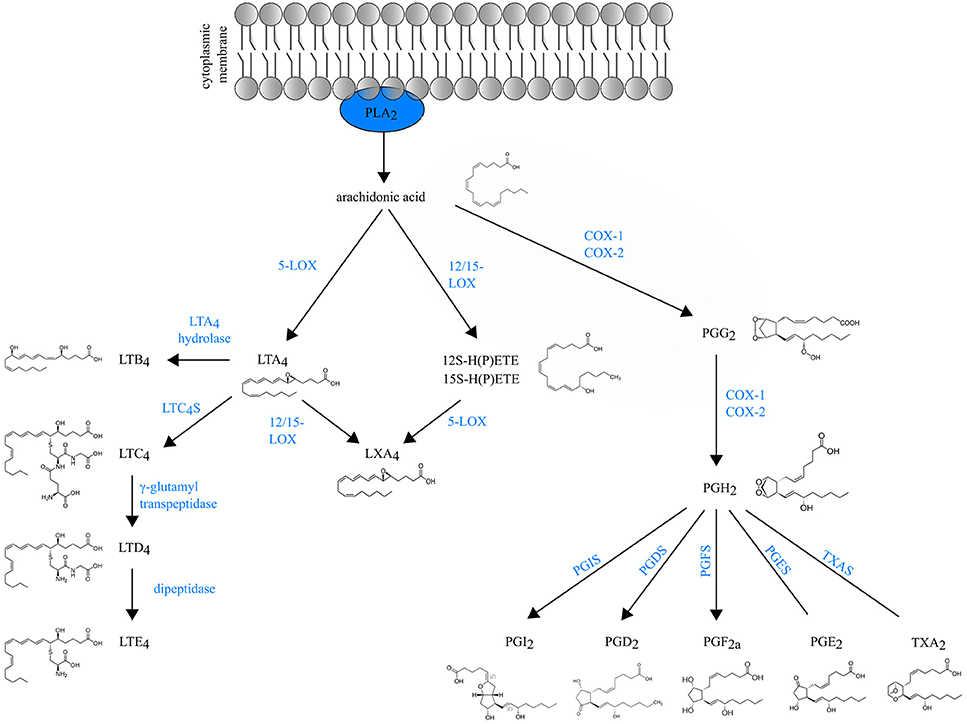
Schematic overview of eicosanoid biosynthesis:
Arachidonic acid released from membrane phospholipids by cytosolic phospholipase A2 can be enzymatically converted either to prostaglandins (PGs) and thromboxane (TXA2) by COX enzymes or to leukotrienes (LTs) and lipoxins (LXA4s) by lipoxygenases (LOXs).
5-LOX: 5-lipoxygenase
12/15-LOX: 12/15-lipoxygenase
LTC4S: LTC4 synthase
PGIS: PGI or prostacyclin synthase
PGDS: PGD2 synthase
PGFS: PGF synthase
PGES: PGE synthase
TXAS: TXA2 synthase
Inflammatory effects of TXA2 in some conditions:
| Prostaglandins | Effects |
|---|---|
| PGD PGD Determination of the nature of a pathological condition or disease in the ovum; zygote; or blastocyst prior to implantation. Cytogenetic analysis is performed to determine the presence or absence of genetic disease. Reproductive Ethical Issues2 (made predominantly by mast cells Mast cells Granulated cells that are found in almost all tissues, most abundantly in the skin and the gastrointestinal tract. Like the basophils, mast cells contain large amounts of histamine and heparin. Unlike basophils, mast cells normally remain in the tissues and do not circulate in the blood. Mast cells, derived from the bone marrow stem cells, are regulated by the stem cell factor. Innate Immunity: Phagocytes and Antigen Presentation) |
|
| PGE1 |
|
| PGE2 |
|
| PGF2ɑ |
|
| PGI PGI An aldose-ketose isomerase that catalyzes the reversible interconversion of glucose 6-phosphate and fructose 6-phosphate. In prokaryotic and eukaryotic organisms it plays an essential role in glycolytic and gluconeogenic pathways. In mammalian systems the enzyme is found in the cytoplasm and as a secreted protein. This secreted form of glucose-6-phosphate isomerase has been referred to as autocrine motility factor or neuroleukin, and acts as a cytokine which binds to the autocrine motility factor receptor. Deficiency of the enzyme in humans is an autosomal recessive trait, which results in congenital nonspherocytic hemolytic anemia. Glycolysis2 (produced by vascular wall endothelial cells) |
|
With multiple biologic effects, several PGs have clinical uses:
Leukotrienes mediate allergic and inflammatory responses with release stimulated by allergens.
| Eicosanoids | Effects |
|---|---|
| LTC4, LTD4, LTE4 |
|
| LTB4 (and HETE) |
|
| LXs A4 and B4 |
|
Leukotrienes are released from cells and their Inflammatory effects are seen in asthma Asthma Asthma is a chronic inflammatory respiratory condition characterized by bronchial hyperresponsiveness and airflow obstruction. The disease is believed to result from the complex interaction of host and environmental factors that increase disease predisposition, with inflammation causing symptoms and structural changes. Patients typically present with wheezing, cough, and dyspnea. Asthma and allergies Allergies A medical specialty concerned with the hypersensitivity of the individual to foreign substances and protection from the resultant infection or disorder. Selective IgA Deficiency.
Inhibition of the pathways, which reduces the production of eicosanoids, also has clinical uses.