Asthma is a chronic inflammatory respiratory condition characterized by bronchial hyperresponsiveness and airflow obstruction. The disease is believed to result from the complex interaction of host and environmental factors that increase disease predisposition, with inflammation Inflammation Inflammation is a complex set of responses to infection and injury involving leukocytes as the principal cellular mediators in the body's defense against pathogenic organisms. Inflammation is also seen as a response to tissue injury in the process of wound healing. The 5 cardinal signs of inflammation are pain, heat, redness, swelling, and loss of function. Inflammation causing symptoms and structural changes. Patients Patients Individuals participating in the health care system for the purpose of receiving therapeutic, diagnostic, or preventive procedures. Clinician–Patient Relationship typically present with wheezing Wheezing Wheezing is an abnormal breath sound characterized by a whistling noise that can be relatively high-pitched and shrill (more common) or coarse. Wheezing is produced by the movement of air through narrowed or compressed small (intrathoracic) airways. Wheezing, cough, and dyspnea Dyspnea Dyspnea is the subjective sensation of breathing discomfort. Dyspnea is a normal manifestation of heavy physical or psychological exertion, but also may be caused by underlying conditions (both pulmonary and extrapulmonary). Dyspnea. Diagnosis is confirmed with a pulmonary function test Pulmonary function test Pulmonary function tests are a group of diagnostic procedures yielding useful, quantifiable information about the rate of the flow of air through the individual's airways, lung capacity, and the efficiency of gas exchange in relation to time. The most commonly utilized tests include spirometry (before and after bronchodilator use), lung volumes, and quantitation of diffusing capacity for carbon monoxide (CO). The tests can be influenced by the individual's effort/fatigue, disease state, or anatomical malformation. Pulmonary Function Tests showing a reversible obstructive pattern. Treatment, based on symptom severity, includes bronchodilators Bronchodilators Asthma Drugs and inhaled corticosteroids Corticosteroids Chorioretinitis for control of inflammation Inflammation Inflammation is a complex set of responses to infection and injury involving leukocytes as the principal cellular mediators in the body's defense against pathogenic organisms. Inflammation is also seen as a response to tissue injury in the process of wound healing. The 5 cardinal signs of inflammation are pain, heat, redness, swelling, and loss of function. Inflammation. Biologic agents Biologic Agents Immunosuppressants targeting inflammatory mediators have been developed for severe persistent asthma Persistent Asthma Asthma in Children.
Last updated: May 17, 2024
Asthma is a chronic inflammatory disorder of the airways:
In established asthma, different triggers may exacerbate the symptoms. These include the following:
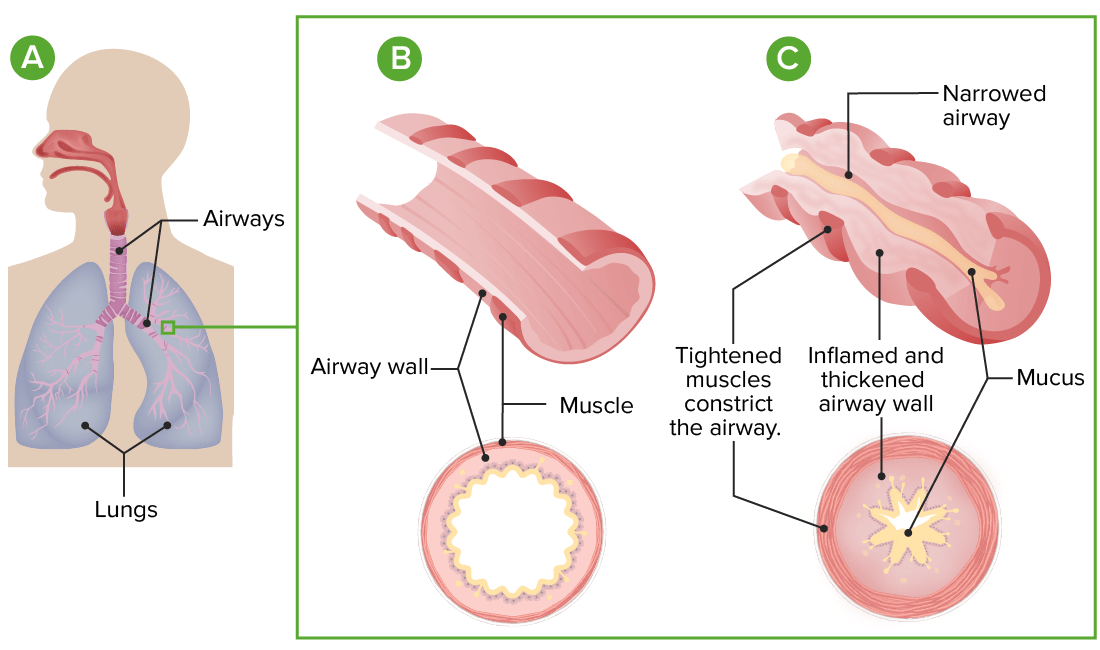
Pathophysiology of asthma:
A: Lung and airway anatomy
B: Cross-section of a normal airway
C: In asthma, exaggerated airway constriction, inflammation, and increased mucus production lead to restriction of airflow.
| Component | Classification | |||
|---|---|---|---|---|
| Intermittent | Persistent: mild | Persistent: moderate | Persistent: severe | |
| Symptoms | ≤ 2 days/week | > 2 but < 7 days/week | Daily | Throughout the day |
| Short-acting bronchodilator use | ≤ 2 days/week | > 2 days/week | Daily | Several times/day |
| Nighttime awakenings | ≤ 2/month | 3–4/month | ≥ 1/week | Nightly |
| Activity limitation | None | Minor | Some | Extreme |
| Lung function | FEV1 > 80% | FEV1 > 80% | FEV1 60%–80% | FEV1 < 60% |
| Exacerbations requiring systemic corticosteroids Corticosteroids Chorioretinitis | 0–1/year | ≥ 2/year | ||
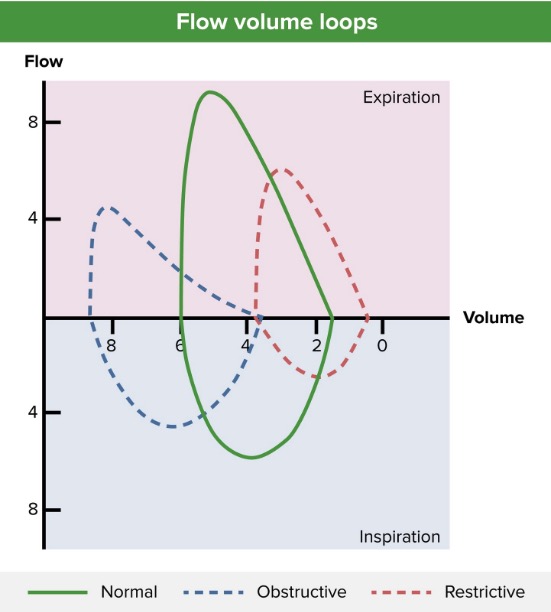
Flow-volume loop (blue line) showing an obstructive pattern of expiration, reduced peak expiratory flow (approximately 4 L/sec), and lung hyperinflation (approximately 4 L at residual volume and > 8 L after full inspiration). A normal pattern (green line) and restrictive pattern (red line) are shown for comparison.
Image by Lecturio.
An individual using an inhaler for asthma (a delivery method for inhaled medications).
Image: “Adult Using an Asthma Inhaler (29251369035)” by National Institute of Allergy and Infectious Diseases (NIAID). License: CC BY 2.0Emergency management is for severe asthma exacerbation, not for responding to initial outpatient management.

Administration of inhaled medications through a nebulizer may be needed for emergent symptom relief.
Image: “Administering inhaled medication” by British Columbia Institute of Technology (BCIT). License: CC BY 4.0