The lincosamides, lincomycin and clindamycin, are inhibitors of bacterial protein synthesis Synthesis Polymerase Chain Reaction (PCR). Drugs in this class share the same binding site as that of macrolides Macrolides Macrolides and ketolides are antibiotics that inhibit bacterial protein synthesis by binding to the 50S ribosomal subunit and blocking transpeptidation. These antibiotics have a broad spectrum of antimicrobial activity but are best known for their coverage of atypical microorganisms. Macrolides and Ketolides and amphenicols; however, they differ in chemical structure. Lincosamides target the 50S ribosomal subunit and interfere with transpeptidation. The antimicrobial coverage of lincosamides encompasses gram-positive Gram-Positive Penicillins cocci Cocci Bacteriology (including MRSA MRSA A strain of Staphylococcus aureus that is non-susceptible to the action of methicillin. The mechanism of resistance usually involves modification of normal or the presence of acquired penicillin binding proteins. Staphylococcus) and anaerobes. Clindamycin can also be used to treat toxic shock syndrome Toxic Shock Syndrome Toxic shock syndrome (TSS) is an acute, multi-systemic disease caused by the toxin-producing bacteria, Staphylococcus aureus and Streptococcus pyogenes. Staphylococcal TSS is more common and associated with tampons and nasal packing. Toxic Shock Syndrome and necrotizing fasciitis Necrotizing fasciitis Necrotizing fasciitis is a life-threatening infection that causes rapid destruction and necrosis of the fascia and subcutaneous tissues. Patients may present with significant pain out of proportion to the presenting symptoms and rapidly progressive erythema of the affected area. Necrotizing Fasciitis owing to its antitoxin effect. Diarrhea Diarrhea Diarrhea is defined as ≥ 3 watery or loose stools in a 24-hour period. There are a multitude of etiologies, which can be classified based on the underlying mechanism of disease. The duration of symptoms (acute or chronic) and characteristics of the stools (e.g., watery, bloody, steatorrheic, mucoid) can help guide further diagnostic evaluation. Diarrhea is a common adverse effect, and clindamycin is often associated with a higher risk of Clostridioides difficile colitis Colitis Inflammation of the colon section of the large intestine, usually with symptoms such as diarrhea (often with blood and mucus), abdominal pain, and fever. Pseudomembranous Colitis.
Last updated: Dec 18, 2024
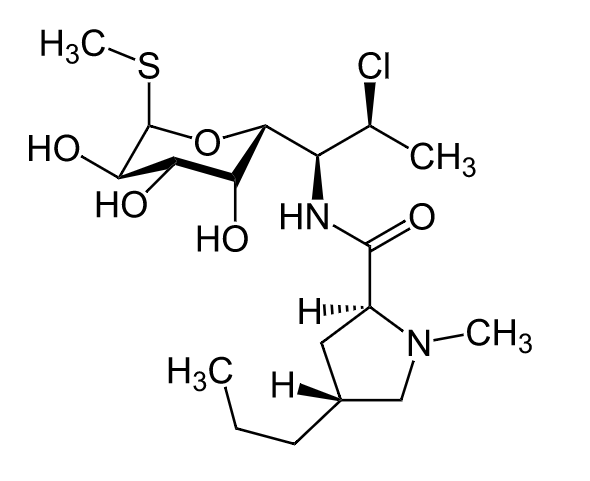
Chemical structure of clindamycin
Image: “Clindamycin” by Jü. License: Public Domain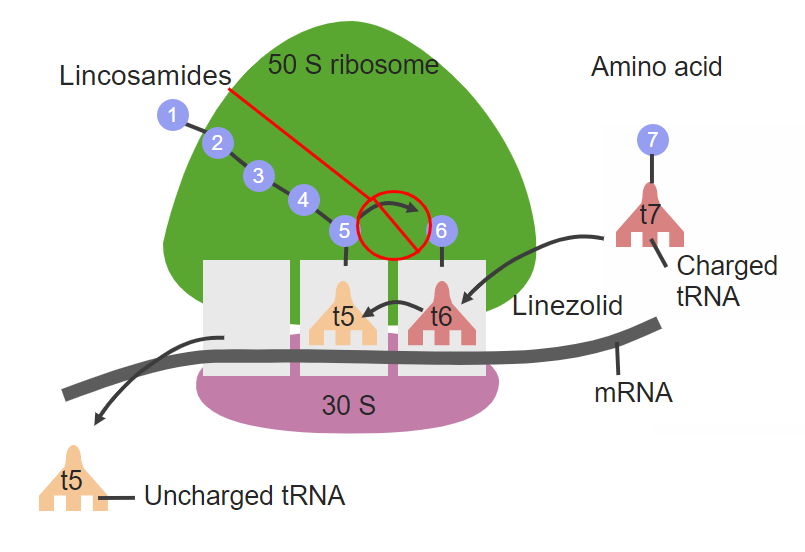
Site of action of clindamycin at the 50S ribosomal subunit
Image by Lecturio. License: CC BY-NC-SA 4.0Since lincomycin is rarely used, the pharmacokinetics Pharmacokinetics Pharmacokinetics is the science that analyzes how the human body interacts with a drug. Pharmacokinetics examines how the drug is absorbed, distributed, metabolized, and excreted by the body. Pharmacokinetics and Pharmacodynamics of clindamycin are have been listed:
There are 3 main routes of resistance Resistance Physiologically, the opposition to flow of air caused by the forces of friction. As a part of pulmonary function testing, it is the ratio of driving pressure to the rate of air flow. Ventilation: Mechanics of Breathing:
The following table compares several classes of bacterial protein synthesis Synthesis Polymerase Chain Reaction (PCR) inhibitor antibiotics:
| Drug class | Mechanism of action | Coverage | Adverse effects |
|---|---|---|---|
| Amphenicols |
|
|
|
| Lincosamides |
|
|
|
| Macrolides Macrolides Macrolides and ketolides are antibiotics that inhibit bacterial protein synthesis by binding to the 50S ribosomal subunit and blocking transpeptidation. These antibiotics have a broad spectrum of antimicrobial activity but are best known for their coverage of atypical microorganisms. Macrolides and Ketolides |
|
|
|
| Oxazolidinones Oxazolidinones The oxazolidinones (linezolid and tedizolid) are bacterial protein synthesis inhibitors. Their unique binding site on the 23S ribosomal RNA of the 50S ribosome gives them zero cross-resistance with other antibiotics. Oxazolidinones |
|
Gram-positive
Gram-Positive
Penicillins
cocci
Cocci
Bacteriology:
|
|
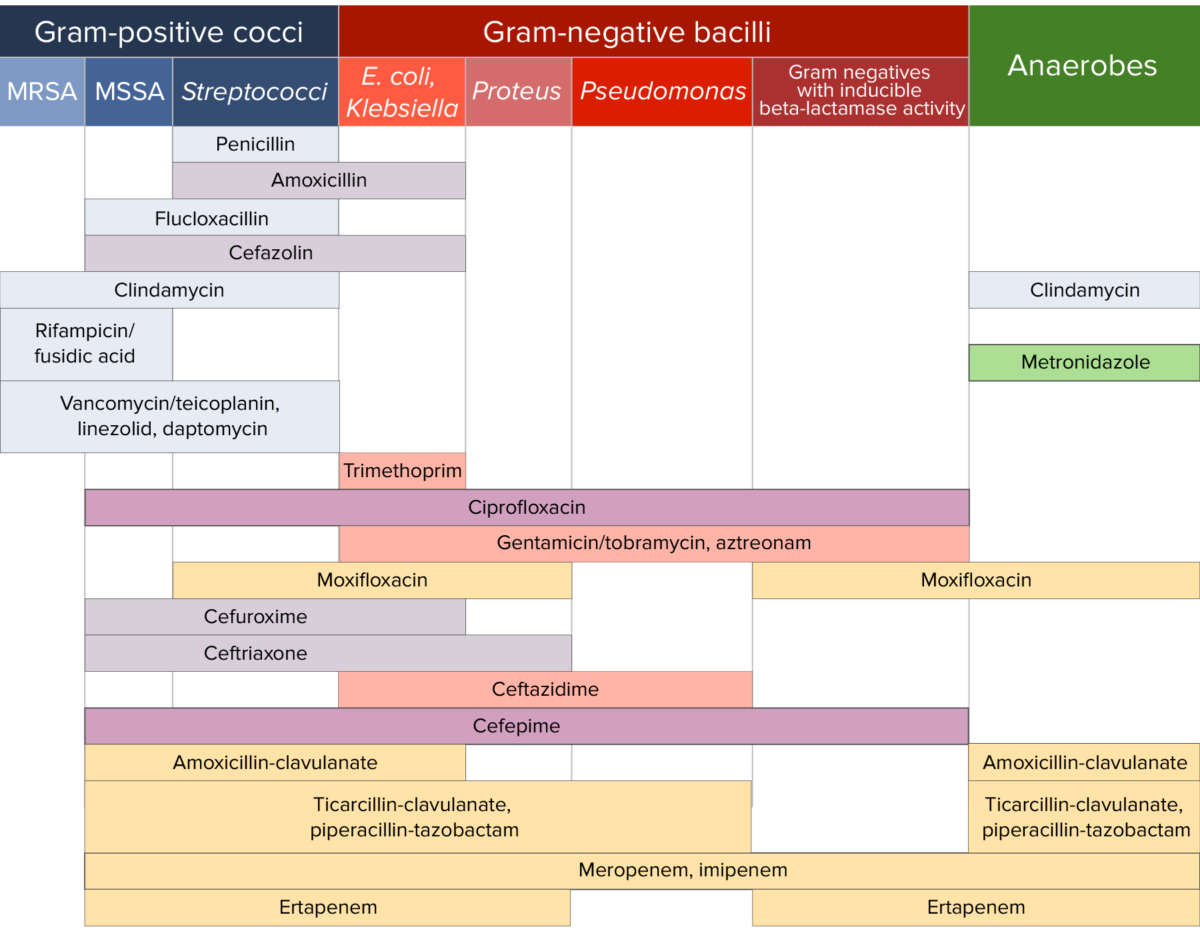
Antibiotic sensitivity:
Chart comparing the microbial coverage of different antibiotics for gram-positive cocci, gram-negative bacilli, and anaerobes.