Advertisement
Advertisement
Advertisement
Advertisement
In addition to the 3 other major classes of antifungal Antifungal Azoles agents ( azoles Azoles Azoles are a widely used class of antifungal medications inhibiting the production of ergosterol, a critical component in the fungal cell membrane. The 2 primary subclasses of azoles are the imidazoles, older agents typically only used for topical applications, and the triazoles, newer agents with a wide spectrum of uses. Azoles, polyenes Polyenes Polyenes are a class of fungicidal agents that consist of 2 primary drugs in current use, namely, nystatin and amphotericin B. Both these drugs exert their effects by binding to ergosterol (a critical component of fungal cell membranes) and creating pores in the membrane, leading to the leakage of intracellular components and ultimately cell lysis. Polyenes, and echinocandins Echinocandins Echinocandins are a group of fungicidal agents that target the fungal cell wall. Echinocandins inhibit β-glucan synthase, which in turn inhibits the production of β-glucan, a key structural component of fungal cell walls. The 3 primary drugs in this class include caspofungin, micafungin, and anidulafungin. Echinocandins), several other clinically important antifungal Antifungal Azoles agents are used, including flucytosine, griseofulvin, and terbinafine. Each drug has a distinct mechanism of action and unique characteristics and indications. Flucytosine is a pyrimidine analog that disrupts fungal DNA DNA A deoxyribonucleotide polymer that is the primary genetic material of all cells. Eukaryotic and prokaryotic organisms normally contain DNA in a double-stranded state, yet several important biological processes transiently involve single-stranded regions. DNA, which consists of a polysugar-phosphate backbone possessing projections of purines (adenine and guanine) and pyrimidines (thymine and cytosine), forms a double helix that is held together by hydrogen bonds between these purines and pyrimidines (adenine to thymine and guanine to cytosine). DNA Types and Structure and RNA RNA A polynucleotide consisting essentially of chains with a repeating backbone of phosphate and ribose units to which nitrogenous bases are attached. RNA is unique among biological macromolecules in that it can encode genetic information, serve as an abundant structural component of cells, and also possesses catalytic activity. RNA Types and Structure synthesis Synthesis Polymerase Chain Reaction (PCR). Flucytosine is always used in combination with other antifungal Antifungal Azoles agents and is primarily used to treat cryptococcal meningitis Cryptococcal meningitis Meningeal inflammation produced by cryptococcus neoformans, an encapsulated yeast that tends to infect individuals with acquired immunodeficiency syndrome and other immunocompromised states. The organism enters the body through the respiratory tract, but symptomatic infections are usually limited to the lungs and nervous system. The organism may also produce parenchymal brain lesions (torulomas). Clinically, the course is subacute and may feature headache; nausea; photophobia; focal neurologic deficits; seizures; cranial neuropathies; and hydrocephalus. Cryptococcus/Cryptococcosis. Both griseofulvin and terbinafine act within the stratum corneum Stratum corneum Skin: Structure and Functions of the skin Skin The skin, also referred to as the integumentary system, is the largest organ of the body. The skin is primarily composed of the epidermis (outer layer) and dermis (deep layer). The epidermis is primarily composed of keratinocytes that undergo rapid turnover, while the dermis contains dense layers of connective tissue. Skin: Structure and Functions and are used to treat dermatophyte infections Infections Invasion of the host organism by microorganisms or their toxins or by parasites that can cause pathological conditions or diseases. Chronic Granulomatous Disease of the skin Skin The skin, also referred to as the integumentary system, is the largest organ of the body. The skin is primarily composed of the epidermis (outer layer) and dermis (deep layer). The epidermis is primarily composed of keratinocytes that undergo rapid turnover, while the dermis contains dense layers of connective tissue. Skin: Structure and Functions, hair, and nails.
Last updated: Feb 28, 2023
Advertisement
Advertisement
Advertisement
Advertisement
Advertisement
Advertisement
Advertisement
Advertisement
In addition to the 3 major classes of antifungal Antifungal Azoles agents ( azoles Azoles Azoles are a widely used class of antifungal medications inhibiting the production of ergosterol, a critical component in the fungal cell membrane. The 2 primary subclasses of azoles are the imidazoles, older agents typically only used for topical applications, and the triazoles, newer agents with a wide spectrum of uses. Azoles, polyenes Polyenes Polyenes are a class of fungicidal agents that consist of 2 primary drugs in current use, namely, nystatin and amphotericin B. Both these drugs exert their effects by binding to ergosterol (a critical component of fungal cell membranes) and creating pores in the membrane, leading to the leakage of intracellular components and ultimately cell lysis. Polyenes, and echinocandins Echinocandins Echinocandins are a group of fungicidal agents that target the fungal cell wall. Echinocandins inhibit β-glucan synthase, which in turn inhibits the production of β-glucan, a key structural component of fungal cell walls. The 3 primary drugs in this class include caspofungin, micafungin, and anidulafungin. Echinocandins), there are several other clinically important antifungals agents. These drugs include:
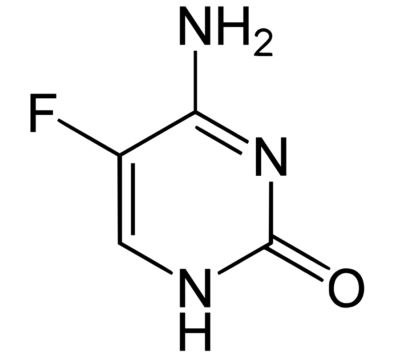
Chemical structure of flucytosine
Image: “Structural diagram of 5-fluorocytosine” by Fvasconcellos. License: Public Domain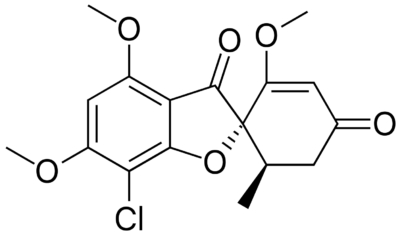
Chemical structure of griseofulvin
Image: “Griseofulvin” by Fvasconcellos. License: Public Domain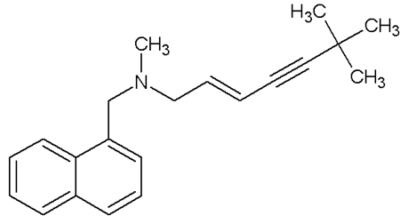
Chemical structure of terbinafine
Image: “Terbinafine” by Rodrica8. License: Public DomainFlucytosine is a pyrimidine analog that disrupts both DNA DNA A deoxyribonucleotide polymer that is the primary genetic material of all cells. Eukaryotic and prokaryotic organisms normally contain DNA in a double-stranded state, yet several important biological processes transiently involve single-stranded regions. DNA, which consists of a polysugar-phosphate backbone possessing projections of purines (adenine and guanine) and pyrimidines (thymine and cytosine), forms a double helix that is held together by hydrogen bonds between these purines and pyrimidines (adenine to thymine and guanine to cytosine). DNA Types and Structure and RNA RNA A polynucleotide consisting essentially of chains with a repeating backbone of phosphate and ribose units to which nitrogenous bases are attached. RNA is unique among biological macromolecules in that it can encode genetic information, serve as an abundant structural component of cells, and also possesses catalytic activity. RNA Types and Structure synthesis Synthesis Polymerase Chain Reaction (PCR).
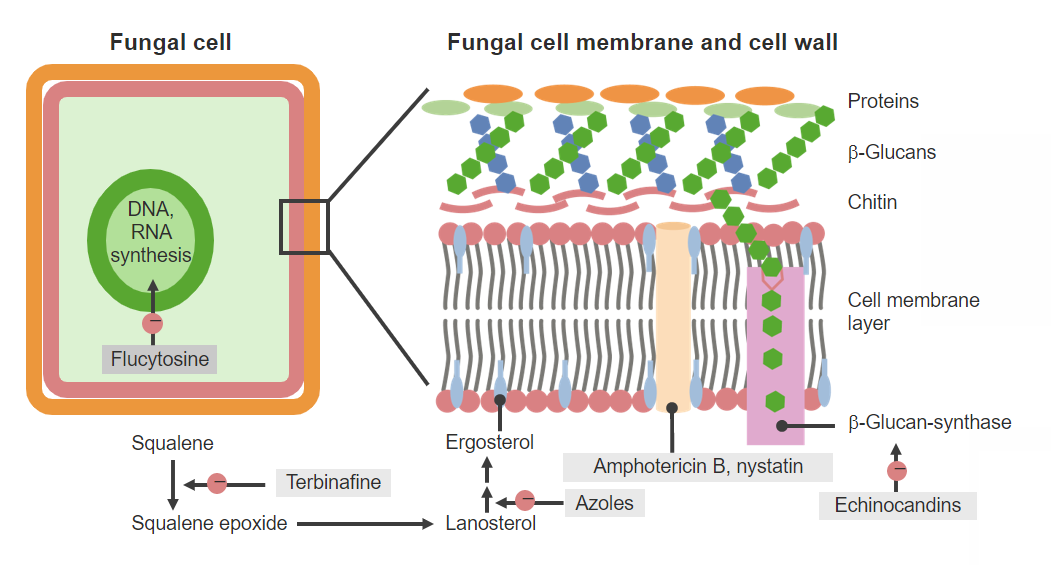
Antifungal agents and mechanisms of action
Image by Lecturio. License: CC BY-NC-SA 4.0The exact mechanisms of griseofulvin are not completely understood, but 2 general mechanisms of action have been suggested:
Terbinafine exerts its effects by causing deterioration of the fungal cell membrane Cell Membrane A cell membrane (also known as the plasma membrane or plasmalemma) is a biological membrane that separates the cell contents from the outside environment. A cell membrane is composed of a phospholipid bilayer and proteins that function to protect cellular DNA and mediate the exchange of ions and molecules. The Cell: Cell Membrane by inhibiting the production of squalene Squalene A natural 30-carbon triterpene. Cholesterol Metabolism epoxide, a precursor to ergosterol Ergosterol A steroid occurring in fungi. Irradiation with ultraviolet rays results in formation of ergocalciferol (vitamin d2). Azoles.
| Drugs | Flucytosine | Griseofulvin | Terbinafine |
|---|---|---|---|
| Absorption Absorption Absorption involves the uptake of nutrient molecules and their transfer from the lumen of the GI tract across the enterocytes and into the interstitial space, where they can be taken up in the venous or lymphatic circulation. Digestion and Absorption |
|
|
|
| Distribution |
|
|
|
| Metabolism | Metabolized intracellularly by yeasts to its active form, 5-FU 5-FU A pyrimidine analog that is an antineoplastic antimetabolite. It interferes with DNA synthesis by blocking the thymidylate synthetase conversion of deoxyuridylic acid to thymidylic acid. Antimetabolite Chemotherapy |
|
|
| Excretion |
|
|
|
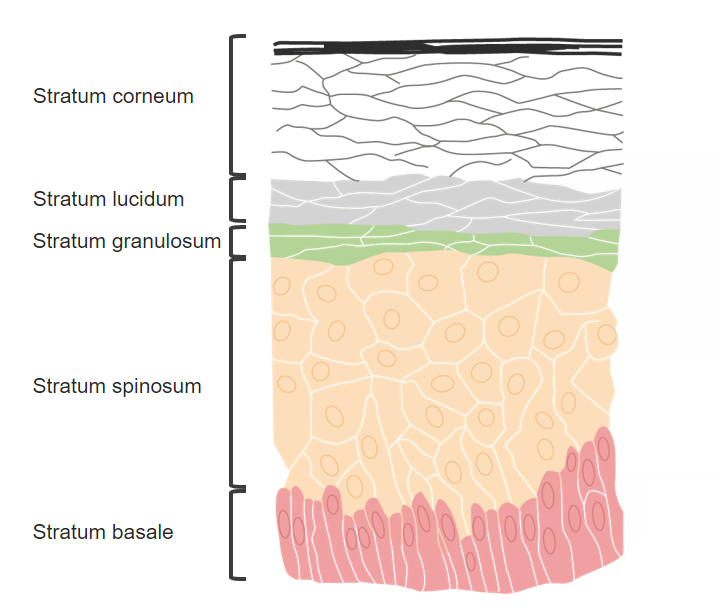
Layers of the skin:
Griseofulvin and terbinafine are distributed to the stratum corneum.
Flucytosine has a narrow spectrum of activity. Owing to its demonstrated synergy with other antifungal Antifungal Azoles agents and the high risk for secondary resistance Resistance Physiologically, the opposition to flow of air caused by the forces of friction. As a part of pulmonary function testing, it is the ratio of driving pressure to the rate of air flow. Ventilation: Mechanics of Breathing when used as monotherapy, flucytosine is not used as a single agent.
Flucytosine is often administered with the highly nephrotoxic agent amphotericin B Amphotericin B Macrolide antifungal antibiotic produced by streptomyces nodosus obtained from soil of the orinoco river region of venezuela. Polyenes. Amphotericin B-induced renal impairment can lead to the accumulation of 5-FC (which is renally cleared) and direct 5-FC toxicity Toxicity Dosage Calculation, which includes:
Griseofulvin is primarily used to treat dermatophyte infections Infections Invasion of the host organism by microorganisms or their toxins or by parasites that can cause pathological conditions or diseases. Chronic Granulomatous Disease of the hair, skin Skin The skin, also referred to as the integumentary system, is the largest organ of the body. The skin is primarily composed of the epidermis (outer layer) and dermis (deep layer). The epidermis is primarily composed of keratinocytes that undergo rapid turnover, while the dermis contains dense layers of connective tissue. Skin: Structure and Functions, or nails. However, griseofulvin is being replaced by newer agents such as terbinafine or itraconazole Itraconazole A triazole antifungal agent that inhibits cytochrome p-450-dependent enzymes required for ergosterol synthesis. Azoles for many of its indications.
Overall, griseofulvin has a relatively low level of toxicity Toxicity Dosage Calculation. Adverse effects may include:
Adverse effects are usually mild and self-limiting Self-Limiting Meningitis in Children.
| Drug class (examples) | Mechanism of action | Clinical relevance |
|---|---|---|
| Azoles Azoles Azoles are a widely used class of antifungal medications inhibiting the production of ergosterol, a critical component in the fungal cell membrane. The 2 primary subclasses of azoles are the imidazoles, older agents typically only used for topical applications, and the triazoles, newer agents with a wide spectrum of uses. Azoles ( Fluconazole Fluconazole Triazole antifungal agent that is used to treat oropharyngeal candidiasis and cryptococcal meningitis in aids. Azoles, Voriconazole Voriconazole A triazole antifungal agent that specifically inhibits sterol 14-alpha-demethylase and cytochrome p-450 cyp3a. Azoles) | Inhibits the production of ergosterol Ergosterol A steroid occurring in fungi. Irradiation with ultraviolet rays results in formation of ergocalciferol (vitamin d2). Azoles (a critical component of the fungal cell membrane Cell Membrane A cell membrane (also known as the plasma membrane or plasmalemma) is a biological membrane that separates the cell contents from the outside environment. A cell membrane is composed of a phospholipid bilayer and proteins that function to protect cellular DNA and mediate the exchange of ions and molecules. The Cell: Cell Membrane) by blocking the lanosterol Lanosterol A triterpene that derives from the chair-boat-chair-boat folding of 2, 3-oxidosqualene. It is metabolized to cholesterol and cucurbitacins. Cholesterol Metabolism 14-α-demethylase enzyme |
|
| Polyenes Polyenes Polyenes are a class of fungicidal agents that consist of 2 primary drugs in current use, namely, nystatin and amphotericin B. Both these drugs exert their effects by binding to ergosterol (a critical component of fungal cell membranes) and creating pores in the membrane, leading to the leakage of intracellular components and ultimately cell lysis. Polyenes ( Amphotericin B Amphotericin B Macrolide antifungal antibiotic produced by streptomyces nodosus obtained from soil of the orinoco river region of venezuela. Polyenes, Nystatin Nystatin Macrolide antifungal antibiotic complex produced by streptomyces noursei, s. Aureus, and other streptomyces species. The biologically active components of the complex are nystatin a1, a2, and a3. Polyenes) | Binds to ergosterol Ergosterol A steroid occurring in fungi. Irradiation with ultraviolet rays results in formation of ergocalciferol (vitamin d2). Azoles in the fungal cell membrane Cell Membrane A cell membrane (also known as the plasma membrane or plasmalemma) is a biological membrane that separates the cell contents from the outside environment. A cell membrane is composed of a phospholipid bilayer and proteins that function to protect cellular DNA and mediate the exchange of ions and molecules. The Cell: Cell Membrane creating artificial pores in the membrane → results in leakage of cellular components and leads to cell lysis (death) |
Amphotericin B
Amphotericin B
Macrolide antifungal antibiotic produced by streptomyces nodosus obtained from soil of the orinoco river region of venezuela.
Polyenes:
Nystatin Nystatin Macrolide antifungal antibiotic complex produced by streptomyces noursei, s. Aureus, and other streptomyces species. The biologically active components of the complex are nystatin a1, a2, and a3. Polyenes:
|
| Echinocandins Echinocandins Echinocandins are a group of fungicidal agents that target the fungal cell wall. Echinocandins inhibit β-glucan synthase, which in turn inhibits the production of β-glucan, a key structural component of fungal cell walls. The 3 primary drugs in this class include caspofungin, micafungin, and anidulafungin. Echinocandins ( Caspofungin Caspofungin A cyclic lipopeptide echinocandin and beta-(1, 3)-d-glucan synthase inhibitor that is used to treat internal or systemic mycoses. Echinocandins, Micafungin Micafungin A cyclic lipo-hexapeptide echinocandin antifungal agent that is used for the treatment and prevention of candidiasis. Echinocandins, Anidulafungin Anidulafungin Echinocandin antifungal agent that is used in the treatment of candidemia and candidiasis. Echinocandins) | Inhibits β-glucan synthase (the enzyme synthesizing β-glucan and an important structural component of the fungal cell wall Fungal Cell Wall Echinocandins) → weakened cell wall Cell wall The outermost layer of a cell in most plants; bacteria; fungi; and algae. The cell wall is usually a rigid structure that lies external to the cell membrane, and provides a protective barrier against physical or chemical agents. Cell Types: Eukaryotic versus Prokaryotic → cell lysis |
|
| Griseofulvin |
|
|
| Terbinafine | Inhibits the squalene Squalene A natural 30-carbon triterpene. Cholesterol Metabolism epoxidase enzyme → blocks the production of squalene Squalene A natural 30-carbon triterpene. Cholesterol Metabolism epoxide, which is a precursor to ergosterol Ergosterol A steroid occurring in fungi. Irradiation with ultraviolet rays results in formation of ergocalciferol (vitamin d2). Azoles and a critical component of the cell membrane Cell Membrane A cell membrane (also known as the plasma membrane or plasmalemma) is a biological membrane that separates the cell contents from the outside environment. A cell membrane is composed of a phospholipid bilayer and proteins that function to protect cellular DNA and mediate the exchange of ions and molecules. The Cell: Cell Membrane |
|
| Flucytosine | A pyrimidine analog with metabolites:
|
|