Echinocandins are a group of fungicidal Fungicidal Polyenes agents that target the fungal cell wall Cell wall The outermost layer of a cell in most plants; bacteria; fungi; and algae. The cell wall is usually a rigid structure that lies external to the cell membrane, and provides a protective barrier against physical or chemical agents. Cell Types: Eukaryotic versus Prokaryotic. Echinocandins inhibit β-glucan synthase, which in turn inhibits the production of β-glucan, a key structural component of fungal cell walls. The 3 primary drugs in this class include caspofungin, micafungin, and anidulafungin. Echinocandins are mainly used to treat Candida Candida Candida is a genus of dimorphic, opportunistic fungi. Candida albicans is part of the normal human flora and is the most common cause of candidiasis. The clinical presentation varies and can include localized mucocutaneous infections (e.g., oropharyngeal, esophageal, intertriginous, and vulvovaginal candidiasis) and invasive disease (e.g., candidemia, intraabdominal abscess, pericarditis, and meningitis). Candida/Candidiasis and Aspergillus infections Infections Invasion of the host organism by microorganisms or their toxins or by parasites that can cause pathological conditions or diseases. Chronic Granulomatous Disease in individuals who are critically ill or have neutropenia Neutropenia Neutrophils are an important component of the immune system and play a significant role in the eradication of infections. Low numbers of circulating neutrophils, referred to as neutropenia, predispose the body to recurrent infections or sepsis, though patients can also be asymptomatic. Neutropenia. Although echinocandins have a narrower spectrum of activity than some other antifungal Antifungal Azoles classes, they are clinically useful because of their relatively low toxicity Toxicity Dosage Calculation profiles and significantly fewer drug interactions than the azoles Azoles Azoles are a widely used class of antifungal medications inhibiting the production of ergosterol, a critical component in the fungal cell membrane. The 2 primary subclasses of azoles are the imidazoles, older agents typically only used for topical applications, and the triazoles, newer agents with a wide spectrum of uses. Azoles and amphotericin B Amphotericin B Macrolide antifungal antibiotic produced by streptomyces nodosus obtained from soil of the orinoco river region of venezuela. Polyenes. Resistance Resistance Physiologically, the opposition to flow of air caused by the forces of friction. As a part of pulmonary function testing, it is the ratio of driving pressure to the rate of air flow. Ventilation: Mechanics of Breathing to echinocandins is generally uncommon, but is emerging in some strains of C. glabrata, usually through mutations that reduce the affinity of this drug to β-glucan synthase.
Last updated: Apr 13, 2025
Echinocandins are a group of antifungal Antifungal Azoles agents that target the fungal cell wall Cell wall The outermost layer of a cell in most plants; bacteria; fungi; and algae. The cell wall is usually a rigid structure that lies external to the cell membrane, and provides a protective barrier against physical or chemical agents. Cell Types: Eukaryotic versus Prokaryotic and are typically used to treat invasive candidiasis Invasive candidiasis An important nosocomial fungal infection with species of the genus candida, most frequently candida albicans. Invasive candidiasis occurs when candidiasis goes beyond a superficial infection and manifests as candidemia, deep tissue infection, or disseminated disease with deep organ involvement. Candida/Candidiasis in individuals with neutropenia Neutropenia Neutrophils are an important component of the immune system and play a significant role in the eradication of infections. Low numbers of circulating neutrophils, referred to as neutropenia, predispose the body to recurrent infections or sepsis, though patients can also be asymptomatic. Neutropenia or those who are critically ill.
Chemical structure of caspofungin
Image: “Caspofungin” by FEERERO. License: Public Domain
Chemical structure of micafungin
Image: “Micafungin” by Fvasconcellos. License: Public Domain
Chemical structure of anidulafungin
Image: “Anidulafungin” by Vaccinationist. License: Public DomainEchinocandins exert their effects by inhibiting fungal cell wall Cell wall The outermost layer of a cell in most plants; bacteria; fungi; and algae. The cell wall is usually a rigid structure that lies external to the cell membrane, and provides a protective barrier against physical or chemical agents. Cell Types: Eukaryotic versus Prokaryotic production by interfering with the synthesis Synthesis Polymerase Chain Reaction (PCR) of β-glucan, an important structural component.
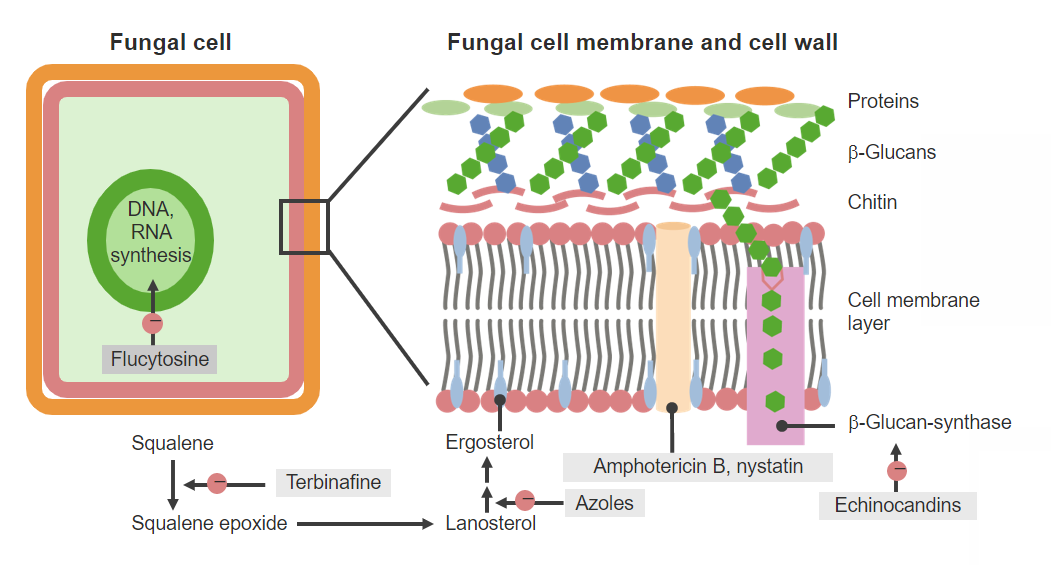
Antifungal agents and mechanisms of action
Image by Lecturio. License: CC BY-NC-SA 4.0Characteristics common to all drugs in this class:
Caspofungin exhibits complicated, triphasic, nonlinear pharmacokinetics Pharmacokinetics Pharmacokinetics is the science that analyzes how the human body interacts with a drug. Pharmacokinetics examines how the drug is absorbed, distributed, metabolized, and excreted by the body. Pharmacokinetics and Pharmacodynamics.
Micafungin exhibits more predictable, linear pharmacokinetics Pharmacokinetics Pharmacokinetics is the science that analyzes how the human body interacts with a drug. Pharmacokinetics examines how the drug is absorbed, distributed, metabolized, and excreted by the body. Pharmacokinetics and Pharmacodynamics.
Anidulafungin exhibits more predictable, linear pharmacokinetics Pharmacokinetics Pharmacokinetics is the science that analyzes how the human body interacts with a drug. Pharmacokinetics examines how the drug is absorbed, distributed, metabolized, and excreted by the body. Pharmacokinetics and Pharmacodynamics.
Rezafungin exhibits predictable, linear pharmacokinetics Pharmacokinetics Pharmacokinetics is the science that analyzes how the human body interacts with a drug. Pharmacokinetics examines how the drug is absorbed, distributed, metabolized, and excreted by the body. Pharmacokinetics and Pharmacodynamics across the therapeutic dose range.
Echinocandins are generally interchangeable with regard to their activity and indications.
Echinocandins are most commonly used in individuals with neutropenia Neutropenia Neutrophils are an important component of the immune system and play a significant role in the eradication of infections. Low numbers of circulating neutrophils, referred to as neutropenia, predispose the body to recurrent infections or sepsis, though patients can also be asymptomatic. Neutropenia or Candida Candida Candida is a genus of dimorphic, opportunistic fungi. Candida albicans is part of the normal human flora and is the most common cause of candidiasis. The clinical presentation varies and can include localized mucocutaneous infections (e.g., oropharyngeal, esophageal, intertriginous, and vulvovaginal candidiasis) and invasive disease (e.g., candidemia, intraabdominal abscess, pericarditis, and meningitis). Candida/Candidiasis infections Infections Invasion of the host organism by microorganisms or their toxins or by parasites that can cause pathological conditions or diseases. Chronic Granulomatous Disease.
Echinocandins are generally very well tolerated and have low toxicity Toxicity Dosage Calculation compared with other antifungal Antifungal Azoles agents. Adverse effects, if seen, may include:
| Drug class (examples) | Mechanism of action | Clinical relevance |
|---|---|---|
| Azoles Azoles Azoles are a widely used class of antifungal medications inhibiting the production of ergosterol, a critical component in the fungal cell membrane. The 2 primary subclasses of azoles are the imidazoles, older agents typically only used for topical applications, and the triazoles, newer agents with a wide spectrum of uses. Azoles ( Fluconazole Fluconazole Triazole antifungal agent that is used to treat oropharyngeal candidiasis and cryptococcal meningitis in aids. Azoles, Voriconazole Voriconazole A triazole antifungal agent that specifically inhibits sterol 14-alpha-demethylase and cytochrome p-450 cyp3a. Azoles) | Inhibits the production of ergosterol Ergosterol A steroid occurring in fungi. Irradiation with ultraviolet rays results in formation of ergocalciferol (vitamin d2). Azoles (a critical component of the fungal cell membrane Cell Membrane A cell membrane (also known as the plasma membrane or plasmalemma) is a biological membrane that separates the cell contents from the outside environment. A cell membrane is composed of a phospholipid bilayer and proteins that function to protect cellular DNA and mediate the exchange of ions and molecules. The Cell: Cell Membrane) by blocking the lanosterol Lanosterol A triterpene that derives from the chair-boat-chair-boat folding of 2, 3-oxidosqualene. It is metabolized to cholesterol and cucurbitacins. Cholesterol Metabolism 14-α-demethylase enzyme |
|
| Polyenes Polyenes Polyenes are a class of fungicidal agents that consist of 2 primary drugs in current use, namely, nystatin and amphotericin B. Both these drugs exert their effects by binding to ergosterol (a critical component of fungal cell membranes) and creating pores in the membrane, leading to the leakage of intracellular components and ultimately cell lysis. Polyenes ( Amphotericin B Amphotericin B Macrolide antifungal antibiotic produced by streptomyces nodosus obtained from soil of the orinoco river region of venezuela. Polyenes, Nystatin Nystatin Macrolide antifungal antibiotic complex produced by streptomyces noursei, s. Aureus, and other streptomyces species. The biologically active components of the complex are nystatin a1, a2, and a3. Polyenes) | Binds to ergosterol Ergosterol A steroid occurring in fungi. Irradiation with ultraviolet rays results in formation of ergocalciferol (vitamin d2). Azoles in the fungal cell membrane Cell Membrane A cell membrane (also known as the plasma membrane or plasmalemma) is a biological membrane that separates the cell contents from the outside environment. A cell membrane is composed of a phospholipid bilayer and proteins that function to protect cellular DNA and mediate the exchange of ions and molecules. The Cell: Cell Membrane creating artificial pores in the membrane → results in leakage of cellular components and leads to cell lysis (death) |
Amphotericin B
Amphotericin B
Macrolide antifungal antibiotic produced by streptomyces nodosus obtained from soil of the orinoco river region of venezuela.
Polyenes:
Nystatin Nystatin Macrolide antifungal antibiotic complex produced by streptomyces noursei, s. Aureus, and other streptomyces species. The biologically active components of the complex are nystatin a1, a2, and a3. Polyenes:
|
| Echinocandins (Caspofungin, Micafungin, Anidulafungin) | Inhibits β-glucan synthase (the enzyme synthesizing β-glucan and an important structural component of the fungal cell wall Cell wall The outermost layer of a cell in most plants; bacteria; fungi; and algae. The cell wall is usually a rigid structure that lies external to the cell membrane, and provides a protective barrier against physical or chemical agents. Cell Types: Eukaryotic versus Prokaryotic) → weakened cell wall Cell wall The outermost layer of a cell in most plants; bacteria; fungi; and algae. The cell wall is usually a rigid structure that lies external to the cell membrane, and provides a protective barrier against physical or chemical agents. Cell Types: Eukaryotic versus Prokaryotic → cell lysis |
|
| Griseofulvin Griseofulvin In addition to the 3 other major classes of antifungal agents (azoles, polyenes, and echinocandins), several other clinically important antifungal agents are used, including flucytosine, griseofulvin, and terbinafine. Griseofulvin acts within the stratum corneum of the skin and are used to treat dermatophyte infections of the skin, hair, and nails. Flucytosine, Griseofulvin, and Terbinafine |
|
|
| Terbinafine Terbinafine In addition to the 3 other major classes of antifungal agents (azoles, polyenes, and echinocandins), several other clinically important antifungal agents are used, including flucytosine, griseofulvin, and terbinafine. Terbinafine acts within the stratum corneum of the skin and are used to treat dermatophyte infections of the skin, hair, and nails. Flucytosine, Griseofulvin, and Terbinafine | Inhibits the squalene epoxidase Squalene Epoxidase Flucytosine, Griseofulvin, and Terbinafine enzyme → blocks the production of squalene epoxide Squalene Epoxide Flucytosine, Griseofulvin, and Terbinafine, which is a precursor to ergosterol Ergosterol A steroid occurring in fungi. Irradiation with ultraviolet rays results in formation of ergocalciferol (vitamin d2). Azoles and a critical component of the cell membrane Cell Membrane A cell membrane (also known as the plasma membrane or plasmalemma) is a biological membrane that separates the cell contents from the outside environment. A cell membrane is composed of a phospholipid bilayer and proteins that function to protect cellular DNA and mediate the exchange of ions and molecules. The Cell: Cell Membrane |
|
| Flucytosine Flucytosine Flucytosine is a pyrimidine analog that disrupts fungal DNA and RNA synthesis. Flucytosine is always used in combination with other antifungal agents and is primarily used to treat cryptococcal meningitis. Flucytosine, Griseofulvin, and Terbinafine | A
pyrimidine analog
Pyrimidine Analog
Flucytosine, Griseofulvin, and Terbinafine with metabolites:
|
|