Antimetabolite chemotherapy Chemotherapy Osteosarcoma agents belong to the cell-cycle–specific drugs, which act on a specific phase of the cell cycle Cell cycle The phases of the cell cycle include interphase (G1, S, and G2) and mitosis (prophase, metaphase, anaphase, and telophase). The cell's progression through these phases is punctuated by checkpoints regulated by cyclins, cyclin-dependent kinases, tumor suppressors, and their antagonists. Cell Cycle. Cancer cells more rapidly divide (or cycle) than normal cells, making them an easy target for chemotherapy Chemotherapy Osteosarcoma. The different cell-cycle phases include G1, S, G2, and M. Antimetabolites target the S phase S Phase Phase of the cell cycle following g1 and preceding g2 when the entire DNA content of the nucleus is replicated. It is achieved by bidirectional replication at multiple sites along each chromosome. Cell Cycle, when DNA replication DNA replication The entire DNA of a cell is replicated during the S (synthesis) phase of the cell cycle. The principle of replication is based on complementary nucleotide base pairing: adenine forms hydrogen bonds with thymine (or uracil in RNA) and guanine forms hydrogen bonds with cytosine. DNA Replication occurs, thus inhibiting DNA DNA A deoxyribonucleotide polymer that is the primary genetic material of all cells. Eukaryotic and prokaryotic organisms normally contain DNA in a double-stranded state, yet several important biological processes transiently involve single-stranded regions. DNA, which consists of a polysugar-phosphate backbone possessing projections of purines (adenine and guanine) and pyrimidines (thymine and cytosine), forms a double helix that is held together by hydrogen bonds between these purines and pyrimidines (adenine to thymine and guanine to cytosine). DNA Types and Structure synthesis Synthesis Polymerase Chain Reaction (PCR) of tumor Tumor Inflammation cells. In this group, the drugs include antifolates (which block folic acid activity, an essential component of DNA DNA A deoxyribonucleotide polymer that is the primary genetic material of all cells. Eukaryotic and prokaryotic organisms normally contain DNA in a double-stranded state, yet several important biological processes transiently involve single-stranded regions. DNA, which consists of a polysugar-phosphate backbone possessing projections of purines (adenine and guanine) and pyrimidines (thymine and cytosine), forms a double helix that is held together by hydrogen bonds between these purines and pyrimidines (adenine to thymine and guanine to cytosine). DNA Types and Structure and RNA RNA A polynucleotide consisting essentially of chains with a repeating backbone of phosphate and ribose units to which nitrogenous bases are attached. RNA is unique among biological macromolecules in that it can encode genetic information, serve as an abundant structural component of cells, and also possesses catalytic activity. RNA Types and Structure precursors), pyrimidine and purine analogs (which interfere with the process of DNA DNA A deoxyribonucleotide polymer that is the primary genetic material of all cells. Eukaryotic and prokaryotic organisms normally contain DNA in a double-stranded state, yet several important biological processes transiently involve single-stranded regions. DNA, which consists of a polysugar-phosphate backbone possessing projections of purines (adenine and guanine) and pyrimidines (thymine and cytosine), forms a double helix that is held together by hydrogen bonds between these purines and pyrimidines (adenine to thymine and guanine to cytosine). DNA Types and Structure synthesis Synthesis Polymerase Chain Reaction (PCR)), and ribonucleotide reductase Ribonucleotide reductase An enzyme of the oxidoreductase class that catalyzes the formation of 2'-deoxyribonucleotides from the corresponding ribonucleotides using NADPH as the ultimate electron donor. The deoxyribonucleoside diphosphates are used in DNA synthesis. Purine and Pyrimidine Metabolism inhibitors (which reduce production of deoxyribonucleotides Deoxyribonucleotides A purine or pyrimidine base bonded to a deoxyribose containing a bond to a phosphate group. DNA Types and Structure). Cell-cycle–specific chemotherapy Chemotherapy Osteosarcoma drugs cannot differentiate healthy from cancerous cells, thus adverse effects are seen. Myelosuppression Myelosuppression Oxazolidinones is a common finding during treatment.
Last updated: Oct 27, 2022
The subtypes of antimetabolite chemotherapy Chemotherapy Osteosarcoma agents are:
| Agent | Mechanism of action | Labeled indications | Adverse effects | Additional considerations |
|---|---|---|---|---|
| Methotrexate | Inhibit DHFR |
|
|
Add leucovorin to rescue cells from toxicity Toxicity Dosage Calculation. |
| Pemetrexed | Inhibit:
|
|
|
Add vitamin B12 and folate Folate Folate and vitamin B12 are 2 of the most clinically important water-soluble vitamins. Deficiencies can present with megaloblastic anemia, GI symptoms, neuropsychiatric symptoms, and adverse pregnancy complications, including neural tube defects. Folate and Vitamin B12 to ↓ toxicity Toxicity Dosage Calculation. |
| Pralatrexate |
|
Peripheral T-cell lymphoma Lymphoma A general term for various neoplastic diseases of the lymphoid tissue. Imaging of the Mediastinum |
|
Add vitamin B12 and folate Folate Folate and vitamin B12 are 2 of the most clinically important water-soluble vitamins. Deficiencies can present with megaloblastic anemia, GI symptoms, neuropsychiatric symptoms, and adverse pregnancy complications, including neural tube defects. Folate and Vitamin B12 to ↓ toxicity Toxicity Dosage Calculation |
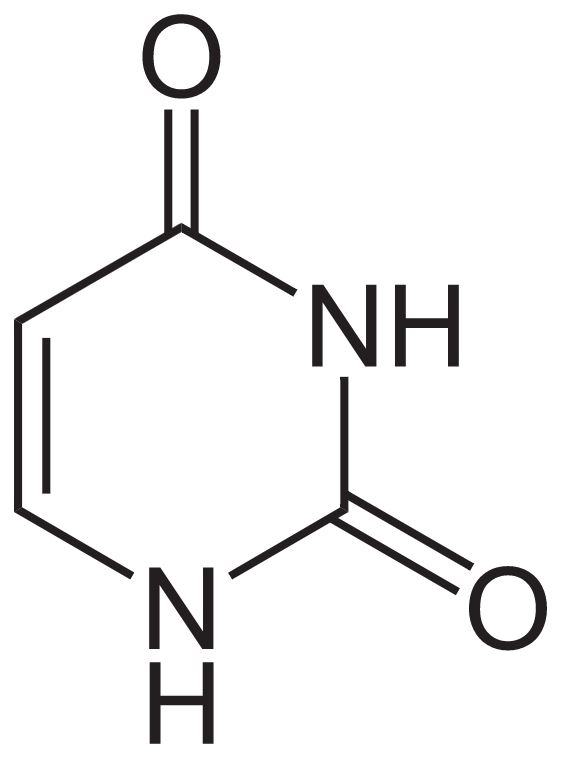
Structure of uracil
Image: “Uracil” by NEUROtiker. License: Public Domain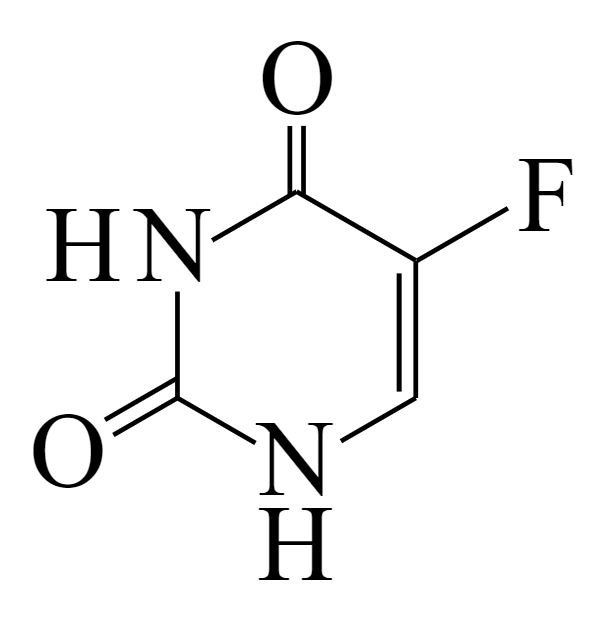
Structure of 5-fluorouracil
Image: “5-Fluorouracil” by すじにくシチュー. License: CC0 1.0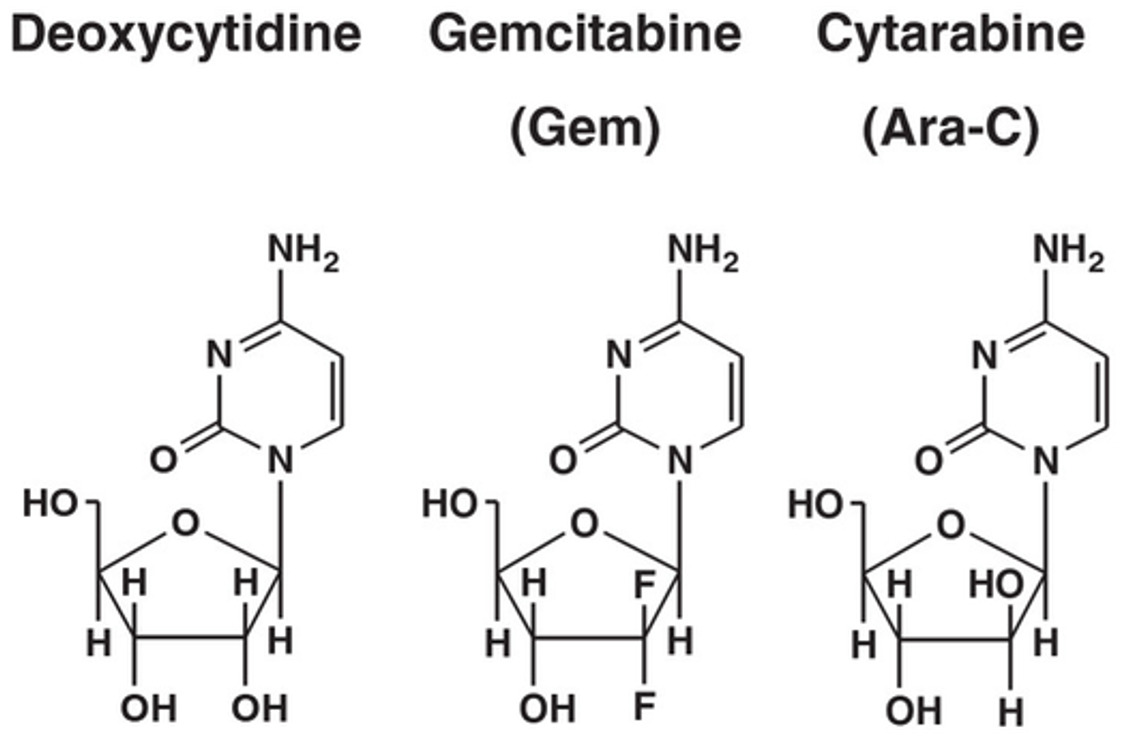
Deoxycytidine and analogs:
Gemcitabine (difluoro analog) and cytarabine (cytosine arabinoside)
| Agent | Mechanism of action | Labeled indications | Adverse effects | Additional considerations |
|---|---|---|---|---|
| 5-FU | Inhibits thymidylate synthetase |
|
|
In DPD deficiency: ↑ toxicity Toxicity Dosage Calculation |
| Capecitabine | Prodrug Prodrug Nitroimidazoles of 5-FU (inhibits thymidylate synthetase) |
|
|
In DPD deficiency: ↑ toxicity Toxicity Dosage Calculation |
| Cytarabine | Inhibits DNA DNA A deoxyribonucleotide polymer that is the primary genetic material of all cells. Eukaryotic and prokaryotic organisms normally contain DNA in a double-stranded state, yet several important biological processes transiently involve single-stranded regions. DNA, which consists of a polysugar-phosphate backbone possessing projections of purines (adenine and guanine) and pyrimidines (thymine and cytosine), forms a double helix that is held together by hydrogen bonds between these purines and pyrimidines (adenine to thymine and guanine to cytosine). DNA Types and Structure polymerase |
|
|
|
| Gemcitabine | Inhibits DNA DNA A deoxyribonucleotide polymer that is the primary genetic material of all cells. Eukaryotic and prokaryotic organisms normally contain DNA in a double-stranded state, yet several important biological processes transiently involve single-stranded regions. DNA, which consists of a polysugar-phosphate backbone possessing projections of purines (adenine and guanine) and pyrimidines (thymine and cytosine), forms a double helix that is held together by hydrogen bonds between these purines and pyrimidines (adenine to thymine and guanine to cytosine). DNA Types and Structure polymerase and ribonucleotide reductase Ribonucleotide reductase An enzyme of the oxidoreductase class that catalyzes the formation of 2′-deoxyribonucleotides from the corresponding ribonucleotides using NADPH as the ultimate electron donor. The deoxyribonucleoside diphosphates are used in DNA synthesis. Purine and Pyrimidine Metabolism |
|
|
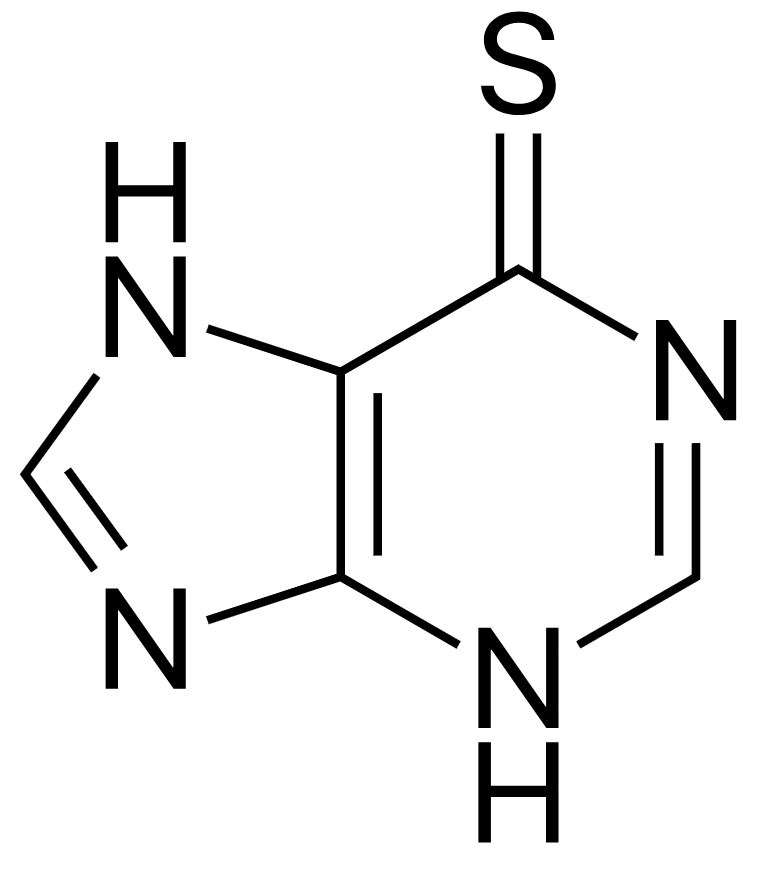
Structure of 6-mercaptopurine
Image: “Mercaptopurine” by Fvasconcellos. License: Public Domain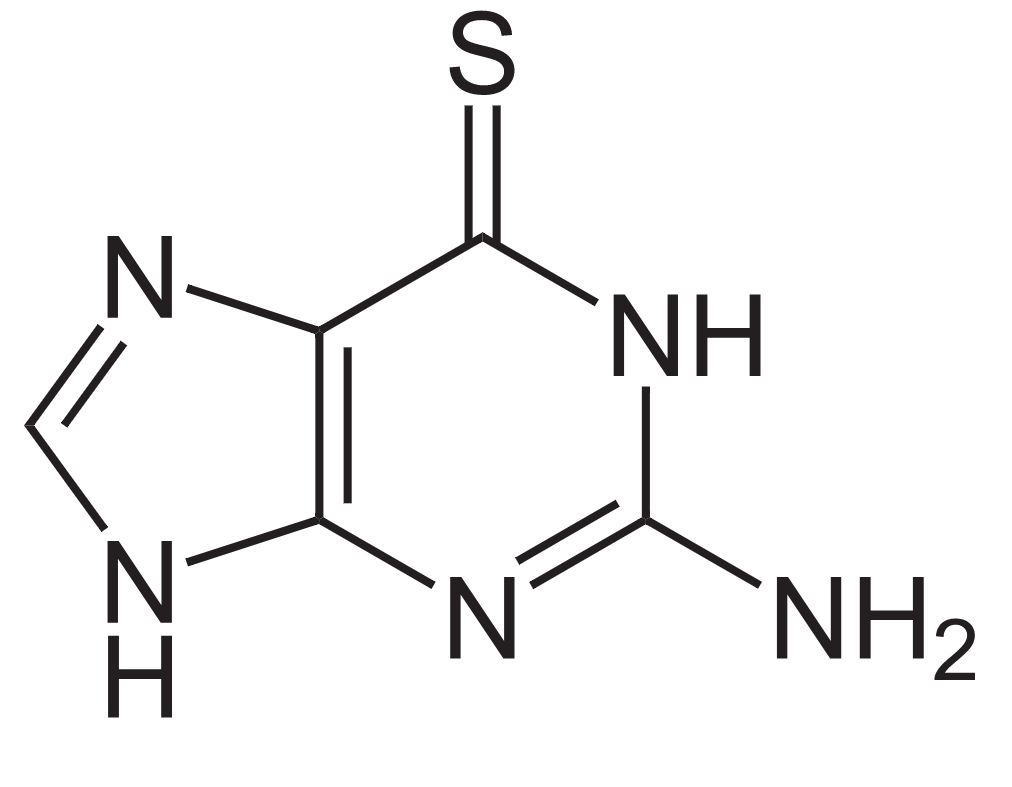
Structure of 6-thioguanine
Image: “6-Thioguanin” by NEUROtiker. License: Public Domain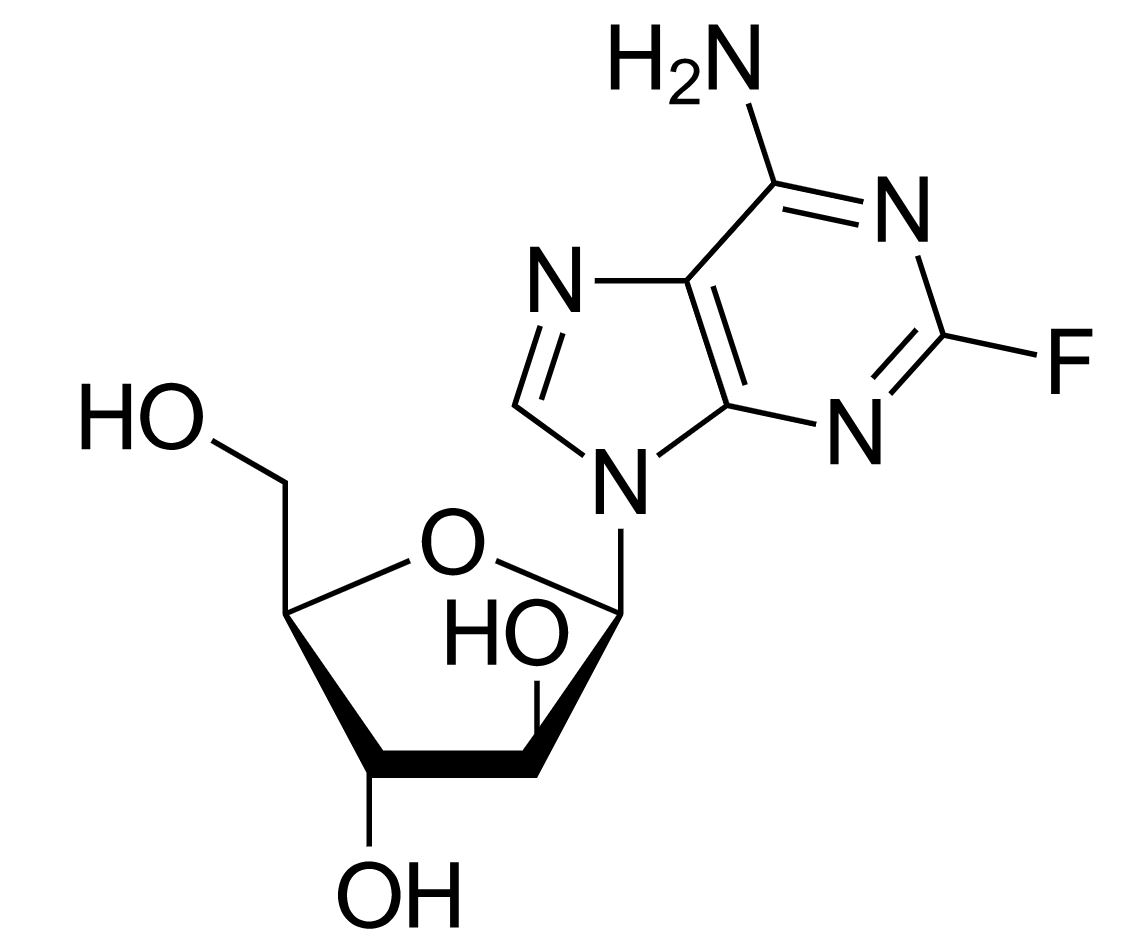
Structure of fludarabine
Image: “Fludarabine” by Yikrazuul. License: Public Domain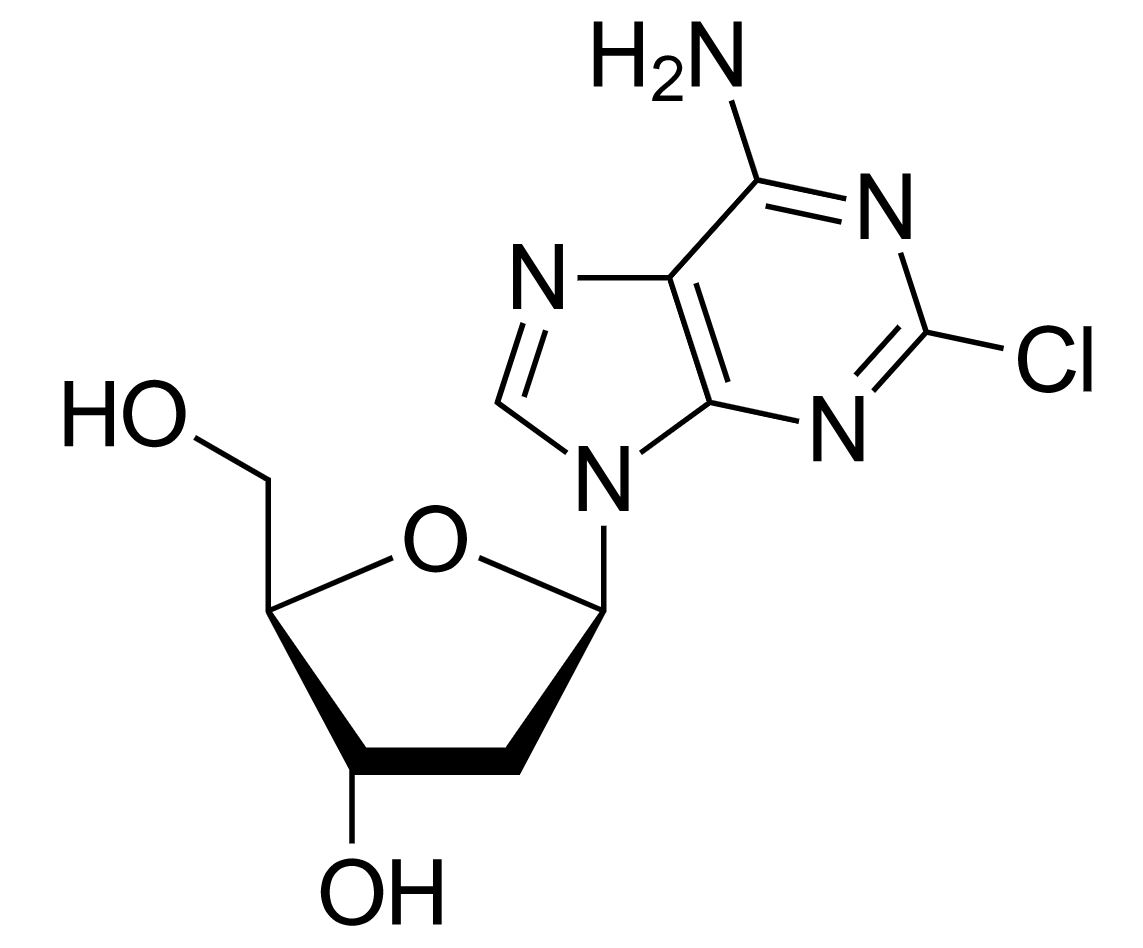
Structure of cladribine
Image: “Cladribine” by Yikrazuul. License: Public Domain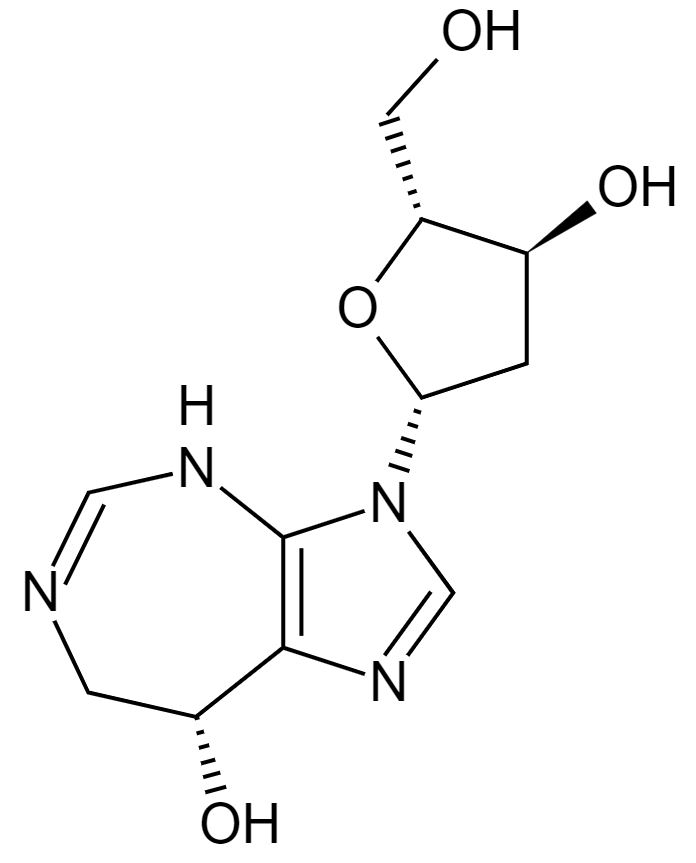
Structure of pentostatin
Image: “Pentostatin” by Fvasconcellos. License: Public Domain| Agent | Mechanism of action | Labeled indications | Adverse effects | Additional considerations |
|---|---|---|---|---|
| 6-MP | Purine antagonist (inhibits purine nucleotide synthesis Synthesis Polymerase Chain Reaction (PCR)) | ALL |
|
↓ Dose if taking allopurinol Allopurinol A xanthine oxidase inhibitor that decreases uric acid production. It also acts as an antimetabolite on some simpler organisms. Gout Drugs |
| 6-TG | Purine antagonist | AML AML Acute myeloid leukemia (AML) is a hematologic malignancy characterized by the uncontrolled proliferation of myeloid precursor cells. Seen predominantly in older adults, AML includes an accumulation of myeloblasts and a replacement of normal marrow by malignant cells, which leads to impaired hematopoiesis. Acute Myeloid Leukemia |
|
|
| Fludarabine | Inhibits:
|
CLL CLL Chronic lymphocytic leukemia (CLL) is a hematologic malignancy characterized by excess production of monoclonal B lymphocytes in the peripheral blood. When the involvement is primarily nodal, the condition is called small lymphocytic lymphoma (SLL). The disease usually presents in older adults, with a median age of 70 years. Chronic Lymphocytic Leukemia |
|
Avoid pentostatin (↑ pulmonary toxicity Toxicity Dosage Calculation) |
| Cladribine | Inhibits:
|
Hairy cell leukemia Hairy cell leukemia Hairy cell leukemia (HCL) is a rare, chronic, B-cell leukemia characterized by the accumulation of small mature B lymphocytes that have “hair-like projections” visible on microscopy. The abnormal cells accumulate in the peripheral blood, bone marrow (causing fibrosis), and red pulp of the spleen, leading to cytopenias. Hairy Cell Leukemia |
|
|
| Pentostatin | Inhibits adenosine Adenosine A nucleoside that is composed of adenine and d-ribose. Adenosine or adenosine derivatives play many important biological roles in addition to being components of DNA and RNA. Adenosine itself is a neurotransmitter. Class 5 Antiarrhythmic Drugs deaminase (↓ DNA DNA A deoxyribonucleotide polymer that is the primary genetic material of all cells. Eukaryotic and prokaryotic organisms normally contain DNA in a double-stranded state, yet several important biological processes transiently involve single-stranded regions. DNA, which consists of a polysugar-phosphate backbone possessing projections of purines (adenine and guanine) and pyrimidines (thymine and cytosine), forms a double helix that is held together by hydrogen bonds between these purines and pyrimidines (adenine to thymine and guanine to cytosine). DNA Types and Structure synthesis Synthesis Polymerase Chain Reaction (PCR)) | Hairy cell leukemia Hairy cell leukemia Hairy cell leukemia (HCL) is a rare, chronic, B-cell leukemia characterized by the accumulation of small mature B lymphocytes that have “hair-like projections” visible on microscopy. The abnormal cells accumulate in the peripheral blood, bone marrow (causing fibrosis), and red pulp of the spleen, leading to cytopenias. Hairy Cell Leukemia |
|
Avoid fludarabine (↑ pulmonary toxicity Toxicity Dosage Calculation) |
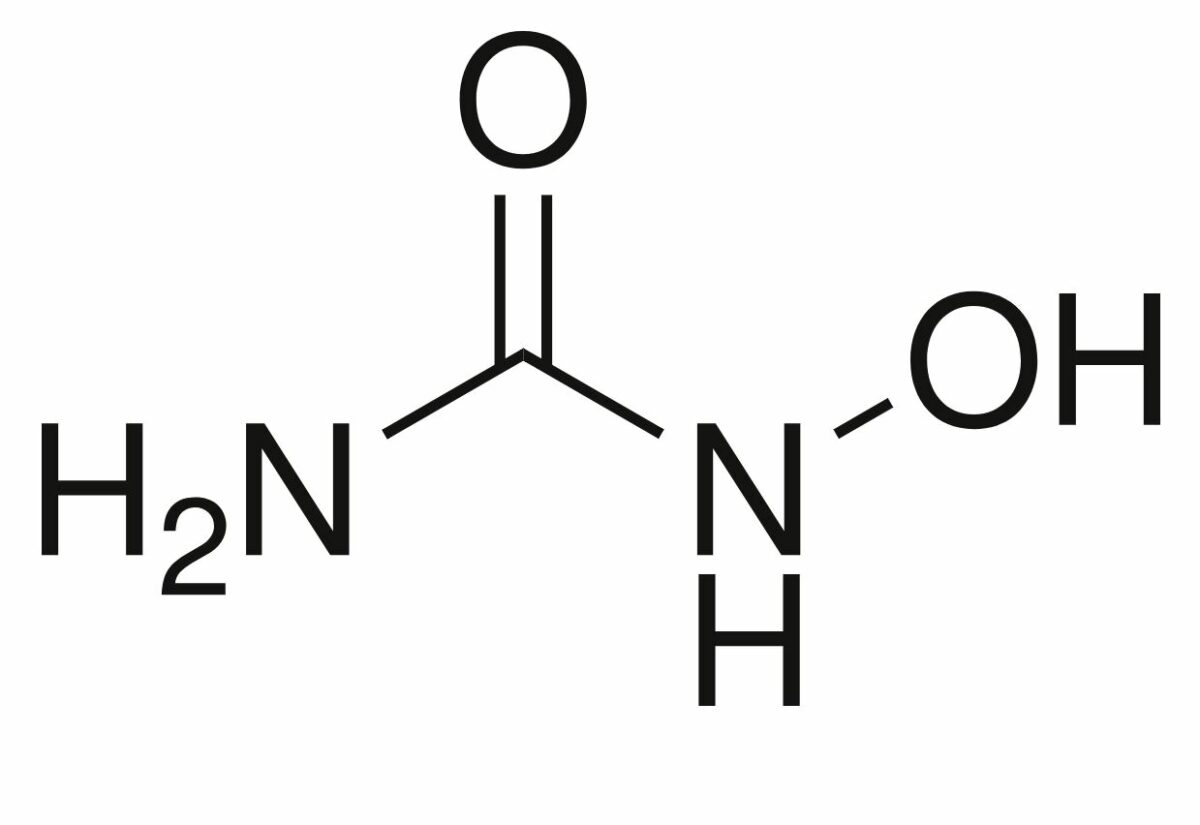
Structure of hydroxyurea
Image: “Hydroxyurea-2D-skeletal” by Chem Sim 2001. License: Public Domain| Drug class | Cell cycle Cell cycle The phases of the cell cycle include interphase (G1, S, and G2) and mitosis (prophase, metaphase, anaphase, and telophase). The cell’s progression through these phases is punctuated by checkpoints regulated by cyclins, cyclin-dependent kinases, tumor suppressors, and their antagonists. Cell Cycle phase affected | Mechanism of action |
|---|---|---|
| Antifolates | Cell cycle Cell cycle The phases of the cell cycle include interphase (G1, S, and G2) and mitosis (prophase, metaphase, anaphase, and telophase). The cell’s progression through these phases is punctuated by checkpoints regulated by cyclins, cyclin-dependent kinases, tumor suppressors, and their antagonists. Cell Cycle arrest at S phase S Phase Phase of the cell cycle following g1 and preceding g2 when the entire DNA content of the nucleus is replicated. It is achieved by bidirectional replication at multiple sites along each chromosome. Cell Cycle | Inhibit:
|
| Fluoropyrimidines | Cell cycle Cell cycle The phases of the cell cycle include interphase (G1, S, and G2) and mitosis (prophase, metaphase, anaphase, and telophase). The cell’s progression through these phases is punctuated by checkpoints regulated by cyclins, cyclin-dependent kinases, tumor suppressors, and their antagonists. Cell Cycle arrest at S phase S Phase Phase of the cell cycle following g1 and preceding g2 when the entire DNA content of the nucleus is replicated. It is achieved by bidirectional replication at multiple sites along each chromosome. Cell Cycle | Inhibit thymidylate synthase Thymidylate synthase An enzyme of the transferase class that catalyzes the reaction 5, 10-methylenetetrahydrofolate and dump to dihydrofolate and dtmp in the synthesis of thymidine triphosphate. Purine and Pyrimidine Metabolism |
| Deoxycytidine analogs | Cell cycle Cell cycle The phases of the cell cycle include interphase (G1, S, and G2) and mitosis (prophase, metaphase, anaphase, and telophase). The cell’s progression through these phases is punctuated by checkpoints regulated by cyclins, cyclin-dependent kinases, tumor suppressors, and their antagonists. Cell Cycle arrest at S phase S Phase Phase of the cell cycle following g1 and preceding g2 when the entire DNA content of the nucleus is replicated. It is achieved by bidirectional replication at multiple sites along each chromosome. Cell Cycle | Inhibit:
|
| Purine analogs | Cell cycle Cell cycle The phases of the cell cycle include interphase (G1, S, and G2) and mitosis (prophase, metaphase, anaphase, and telophase). The cell’s progression through these phases is punctuated by checkpoints regulated by cyclins, cyclin-dependent kinases, tumor suppressors, and their antagonists. Cell Cycle arrest at S phase S Phase Phase of the cell cycle following g1 and preceding g2 when the entire DNA content of the nucleus is replicated. It is achieved by bidirectional replication at multiple sites along each chromosome. Cell Cycle | Inhibition of de novo purine synthesis Synthesis Polymerase Chain Reaction (PCR) |
| Topoisomerase II Topoisomerase II DNA topoisomerases that catalyze ATP-dependent breakage of both strands of DNA, passage of the unbroken strands through the breaks, and rejoining of the broken strands. These enzymes bring about relaxation of the supercoiled DNA and resolution of a knotted circular DNA duplex. Fluoroquinolones inhibitors | Cell cycle Cell cycle The phases of the cell cycle include interphase (G1, S, and G2) and mitosis (prophase, metaphase, anaphase, and telophase). The cell’s progression through these phases is punctuated by checkpoints regulated by cyclins, cyclin-dependent kinases, tumor suppressors, and their antagonists. Cell Cycle arrest at S and G2 phases | Inhibit topoisomerase II Topoisomerase II DNA topoisomerases that catalyze ATP-dependent breakage of both strands of DNA, passage of the unbroken strands through the breaks, and rejoining of the broken strands. These enzymes bring about relaxation of the supercoiled DNA and resolution of a knotted circular DNA duplex. Fluoroquinolones |
| Taxanes Taxanes A group of diterpenoid cyclodecanes named for the taxanes that were discovered in the taxus tree. The action on microtubules has made some of them useful as antineoplastic agents. Microtubule and Topoisomerase Inhibitors | Cell cycle Cell cycle The phases of the cell cycle include interphase (G1, S, and G2) and mitosis (prophase, metaphase, anaphase, and telophase). The cell’s progression through these phases is punctuated by checkpoints regulated by cyclins, cyclin-dependent kinases, tumor suppressors, and their antagonists. Cell Cycle arrest at metaphase Metaphase The phase of cell nucleus division following prometaphase, in which the chromosomes line up across the equatorial plane of the spindle apparatus prior to separation. Cell Cycle of the M phase | Hyper-stabilization of microtubules Microtubules Slender, cylindrical filaments found in the cytoskeleton of plant and animal cells. They are composed of the protein tubulin and are influenced by tubulin modulators. The Cell: Cytosol and Cytoskeleton |
| Vinca alkaloids Vinca Alkaloids A group of indole-indoline dimers which are alkaloids obtained from the vinca genus of plants. They inhibit polymerization of tubulin into microtubules thus blocking spindle formation and arresting cells in metaphase. They are some of the most useful antineoplastic agents. Microtubule and Topoisomerase Inhibitors | Cell arrest during metaphase Metaphase The phase of cell nucleus division following prometaphase, in which the chromosomes line up across the equatorial plane of the spindle apparatus prior to separation. Cell Cycle of the M phase | Binds to beta-tubulin and prevents microtubule polymerization |