Gastric cancer is the 3rd-most common cause of cancer-related deaths worldwide. The majority of cases are from adenocarcinoma. The modifiable risk factors include Helicobacter pylori Helicobacter pylori A spiral bacterium active as a human gastric pathogen. It is a gram-negative, urease-positive, curved or slightly spiral organism initially isolated in 1982 from patients with lesions of gastritis or peptic ulcers in Western Australia. Helicobacter pylori was originally classified in the genus campylobacter, but RNA sequencing, cellular fatty acid profiles, growth patterns, and other taxonomic characteristics indicate that the micro-organism should be included in the genus Helicobacter. It has been officially transferred to Helicobacter gen. Helicobacter infection, smoking Smoking Willful or deliberate act of inhaling and exhaling smoke from burning substances or agents held by hand. Interstitial Lung Diseases, and nitrate-rich diets. Hereditary syndromes, pernicious anemia Pernicious anemia A megaloblastic anemia occurring in children but more commonly in later life, characterized by histamine-fast achlorhydria, in which the laboratory and clinical manifestations are based on malabsorption of vitamin B12 due to a failure of the gastric mucosa to secrete adequate and potent intrinsic factor. Megaloblastic Anemia, and prior partial gastrectomy are among the endogenous risk factors. When symptoms such as epigastric fullness, vomiting Vomiting The forcible expulsion of the contents of the stomach through the mouth. Hypokalemia, and weight loss Weight loss Decrease in existing body weight. Bariatric Surgery occur, it is likely that the cancer is in the advanced stage. Diagnosis is confirmed with esophagogastroduodenoscopy and biopsy Biopsy Removal and pathologic examination of specimens from the living body. Ewing Sarcoma. Imaging studies and laparoscopy Laparoscopy Laparoscopy is surgical exploration and interventions performed through small incisions with a camera and long instruments. Laparotomy and Laparoscopy aid in determining the cancer stage. Consequently, staging Staging Methods which attempt to express in replicable terms the extent of the neoplasm in the patient. Grading, Staging, and Metastasis dictates the management approach. Management consists of gastrectomy and chemoradiotherapy. Most cases are diagnosed in late stages, indicating a generally poor prognosis Prognosis A prediction of the probable outcome of a disease based on a individual's condition and the usual course of the disease as seen in similar situations. Non-Hodgkin Lymphomas.
Last updated: Dec 5, 2022
Endogenous risk factors:
Exogenous risk factors:
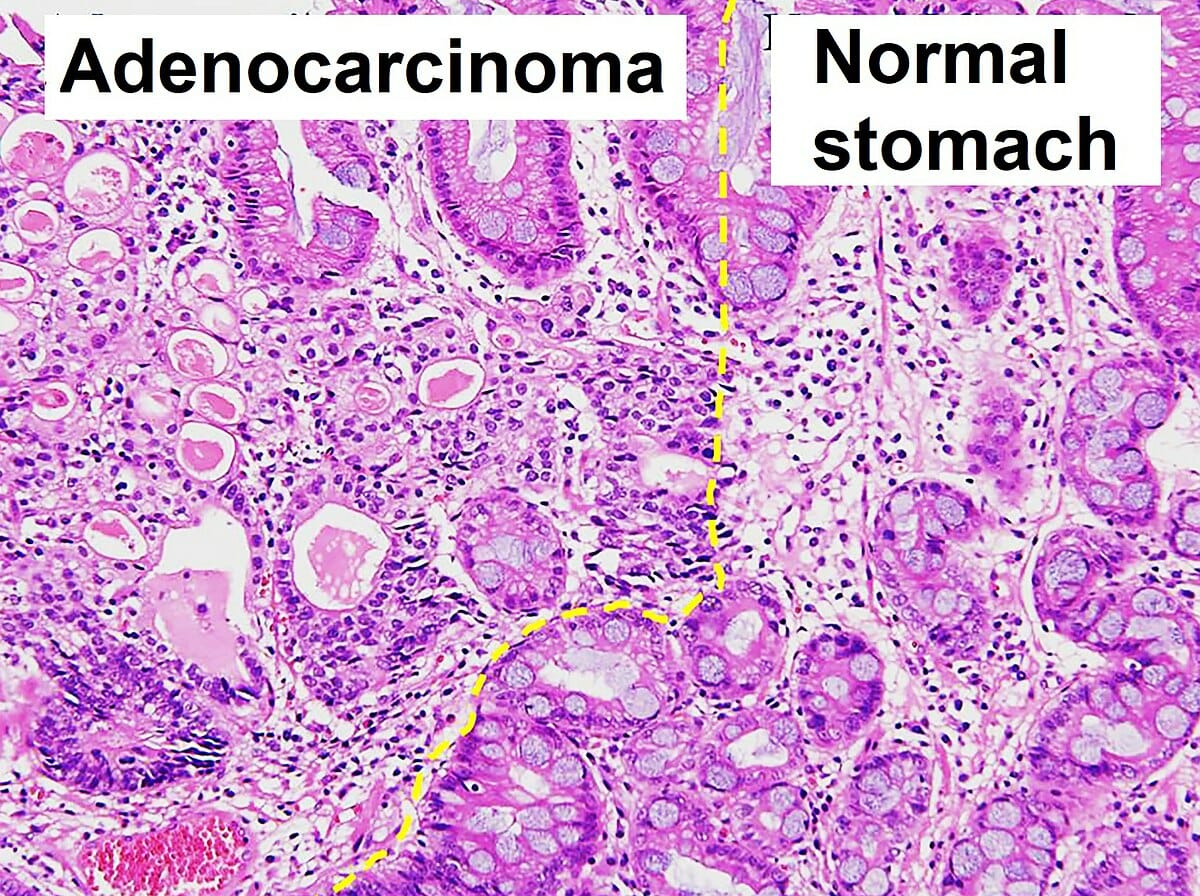
Histopathology of gastric adenocarcinoma and normal pathology
Image: “Histopathology of gastric adenocarcinoma and normal histology” by Ji Min Choi et al. License: CC BY 4.0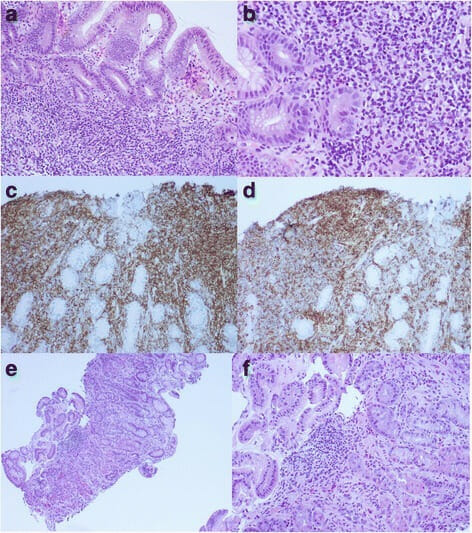
Gastric mucosa-associated lymphoid tissue lymphoma and Helicobacter pylori infection:
a. Gastric biopsy shows intralaminar small lymphocytic infiltrate with monotonous features (H&E stain, magnification 100x).
b. Foci of lymphoepithelial lesion (H&E stain, magnification 200x)
c. Sheets of CD20+ small B cells seen on CD20 immunostain (magnification 200x)
d. Immunostaining also reveals aberrant expression of CD43 (magnification 200x).
e. Repeat gastric biopsy 6 months after H. pylori eradication shows scattered intralaminar infiltrates of small lymphocytes, plasma cells, and a few small, loose lymphoid aggregates, indicating complete remission (H&E stain, magnification 100x).
f. The same findings are seen at 200x magnification (H&E stain).
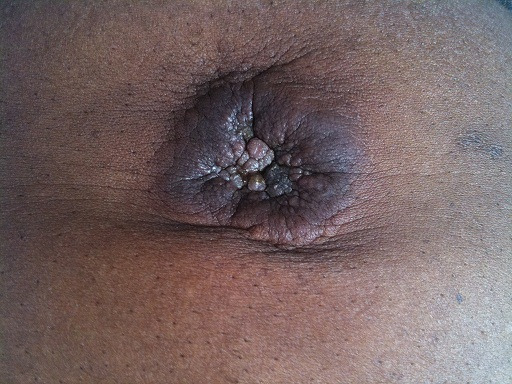
Sister Mary Joseph’s nodule in a patient with metastatic gastrointestinal tract cancer
Image: “Sister Mary Joseph nodule” by Touré PS, Tall CT, Dioussé P, Berthé A, Diop MM, Sarr MM, Diop B, Léye YM, Diop BM, Ka MM. License: CC BY 2.0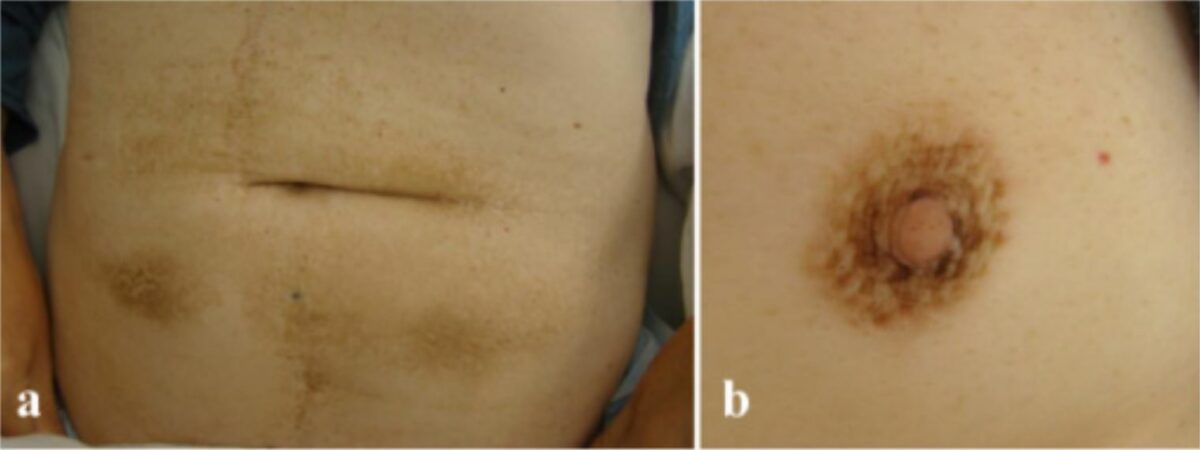
Acanthosis nigricans on a) the abdomen and b) the areola
Image: “Malignant acanthosis nigricans associated with prostate cancer” by Kubicka-Wołkowska J, Dębska-Szmich S, Lisik-Habib M, Noweta M, Potemski P. License: CC BY 4.0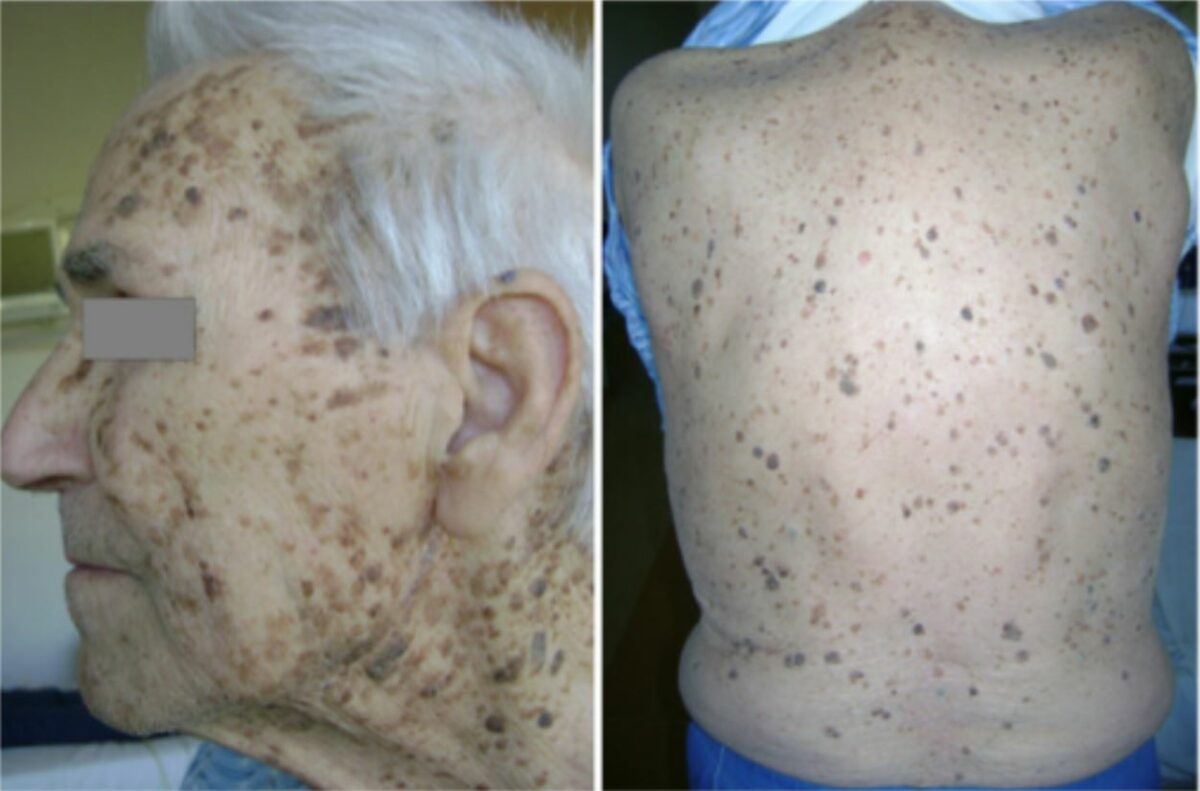
Patient with occult gastric adenocarcinoma: innumerable seborrheic keratoses concentrated over the face, neck, back, and chest.
Image: “Leser-Trélat syndrome” by Ponti G, Luppi G, Losi L, Giannetti A, Seidenari S. License: CC BY 2.0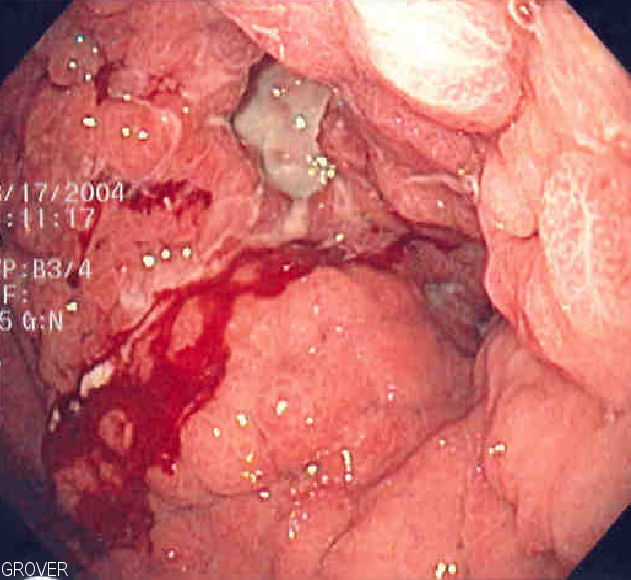
Endoscopic image of linitis plastica, where the entire stomach is invaded with cancer, leading to a leather-bottle appearance
Image: “Linitis plastica” by Samir. License: Public Domain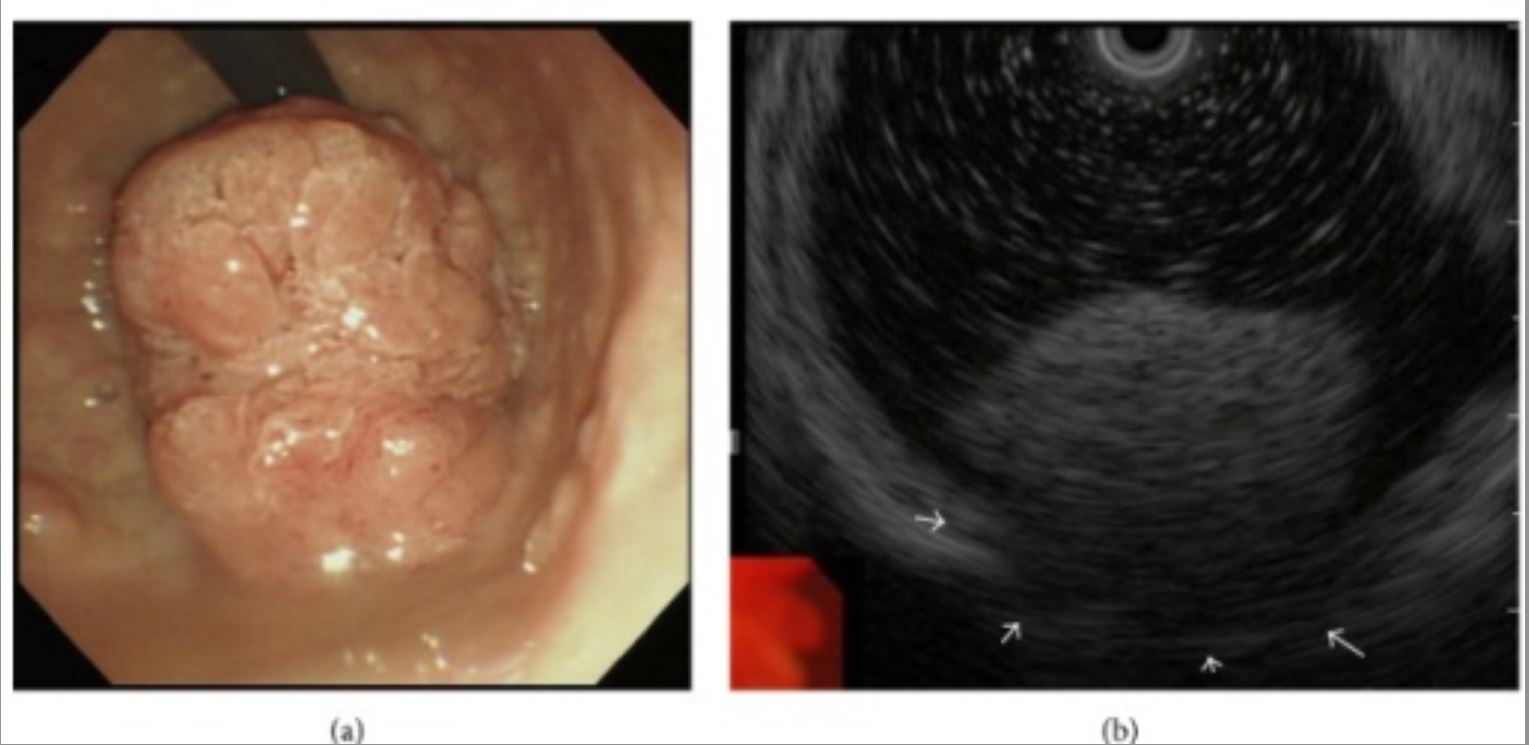
Polypoid tumor expressing on the pyloric ring:
a: Upper gastric endoscopy reveals a giant polypoid-type tumor extending from the duodenum bulb to the pyloric ring.
b: Endoscopic US suggests tumor invasion of the muscular tunic (white arrows).
| T (primary tumor Tumor Inflammation) | N ( lymph nodes Lymph Nodes They are oval or bean shaped bodies (1 – 30 mm in diameter) located along the lymphatic system. Lymphatic Drainage System: Anatomy affected) | M (distant metastases) | |||
|---|---|---|---|---|---|
| TX | Cannot be assessed | N0 | No regional lymph nodes Lymph Nodes They are oval or bean shaped bodies (1 – 30 mm in diameter) located along the lymphatic system. Lymphatic Drainage System: Anatomy | M0 | No distant metastases |
| T0 | No evidence of tumor Tumor Inflammation | N1 | 1–2 regional nodes | M1 | Confirmed metastases |
| Tis | Carcinoma in situ Carcinoma in situ A lesion with cytological characteristics associated with invasive carcinoma but the tumor cells are confined to the epithelium of origin, without invasion of the basement membrane. Leukoplakia | N2 | 3–6 regional nodes | ||
| T1 | Invasion up to submucosa | N3 | 7 or more regional nodes | ||
| T2 | Invasion of muscularis propria | ||||
| T3 T3 A T3 thyroid hormone normally synthesized and secreted by the thyroid gland in much smaller quantities than thyroxine (T4). Most T3 is derived from peripheral monodeiodination of T4 at the 5′ position of the outer ring of the iodothyronine nucleus. The hormone finally delivered and used by the tissues is mainly t3. Thyroid Hormones | Invasion of serosa | ||||
| T4a | Perforates serosa | ||||
| T4b | Adjacent structures affected | ||||
| Stage | TNM | Features |
|---|---|---|
| 0 | TisN0M0 | Node negative; limited to mucosa |
| 1 | T1-2N0M0 | Node negative; invasion of submucosa up to muscularis propria |
| 2a | T1N1-3M0 T2N1-3M |
Node positive; invasion of muscularis propria |
| 2b | T3N0M0 T4aN0M0 |
Node negative; invasion up to serosa |
| 3 |
T3
T3
A T3 thyroid hormone normally synthesized and secreted by the thyroid gland in much smaller quantities than thyroxine (T4). Most T3 is derived from peripheral monodeiodination of T4 at the 5′ position of the outer ring of the iodothyronine nucleus. The hormone finally delivered and used by the tissues is mainly t3.
Thyroid Hormones,N1-3,M0 T4a,N1-3, M0 |
Node positive; invasion up to serosa |
| 4a | T4b, any N, M0 | Node positive; beyond serosa, up to adjacent structures |
| 4b | Any T, N, M1 | Distant metastases |
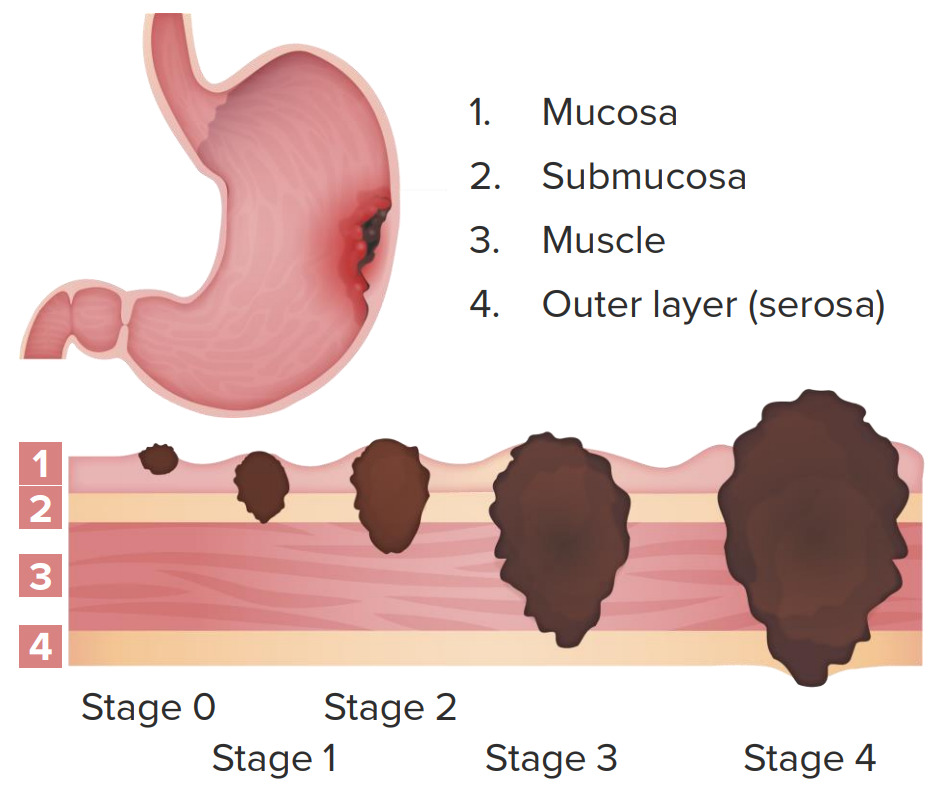
Clinical staging of gastric cancer:
Stage 0: node negative; limited to mucosa
Stage 1: node negative; invasion of submucosa and part of muscularis propria
Stage 2: node negative with invasion up to serosa or node positive with invasion up to muscularis propria
Stage 3: node positive; invasion of serosa
Stage 4: node positive; invasion up to adjacent structures with or without distant metastasis