Tumor Tumor Inflammation lysis syndrome is a potentially lethal group of metabolic disturbances that occurs when large numbers of cancer cells are killed rapidly. The lysed cells release their intracellular contents into the bloodstream, resulting in the development of hyperkalemia Hyperkalemia Hyperkalemia is defined as a serum potassium (K+) concentration >5.2 mEq/L. Homeostatic mechanisms maintain the serum K+ concentration between 3.5 and 5.2 mEq/L, despite marked variation in dietary intake. Hyperkalemia can be due to a variety of causes, which include transcellular shifts, tissue breakdown, inadequate renal excretion, and drugs. Hyperkalemia, hyperuricemia Hyperuricemia Excessive uric acid or urate in blood as defined by its solubility in plasma at 37 degrees c; greater than 0. 42 mmol per liter (7. 0 mg/dl) in men or 0. 36 mmol per liter (6. 0 mg/dl) in women. Gout, hyperphosphatemia Hyperphosphatemia A condition of abnormally high level of phosphates in the blood, usually significantly above the normal range of 0. 84-1. 58 mmol per liter of serum. Hypocalcemia, hypocalcemia Hypocalcemia Hypocalcemia, a serum calcium < 8.5 mg/dL, can result from various conditions. The causes may include hypoparathyroidism, drugs, disorders leading to vitamin D deficiency, and more. Calcium levels are regulated and affected by different elements such as dietary intake, parathyroid hormone (PTH), vitamin D, pH, and albumin. Presentation can range from an asymptomatic (mild deficiency) to a life-threatening condition (acute, significant deficiency). Hypocalcemia, and acute kidney injury Acute Kidney Injury Acute kidney injury refers to sudden and often reversible loss of renal function, which develops over days or weeks. Azotemia refers to elevated levels of nitrogen-containing substances in the blood that accompany AKI, which include BUN and creatinine. Acute Kidney Injury. This can lead to severe neurologic, cardiac, gastrointestinal, and urinary signs and symptoms. The diagnosis is made based on the metabolic derangements seen on laboratory evaluation in conjunction with the clinical history. In addressing tumor Tumor Inflammation lysis syndrome, the goal is to initiate therapy early for those at high risk, including IV hydration Iv Hydration Crush Syndrome, close electrolyte monitoring and correction, and hypouricemic agents.
Last updated: Feb 28, 2025
National incidence Incidence The number of new cases of a given disease during a given period in a specified population. It also is used for the rate at which new events occur in a defined population. It is differentiated from prevalence, which refers to all cases in the population at a given time. Measures of Disease Frequency reports are hampered by a lack of standard criteria for diagnosis.
Tumor Tumor Inflammation lysis syndrome results from massive tumor Tumor Inflammation death and is primarily associated with the following hematologic malignancies:
Malignancy Malignancy Hemothorax characteristics:
Concurrent conditions:
Tumor Tumor Inflammation lysis syndrome occurs secondary to the chemotherapeutic treatment of malignancies, resulting in massive cell destruction.
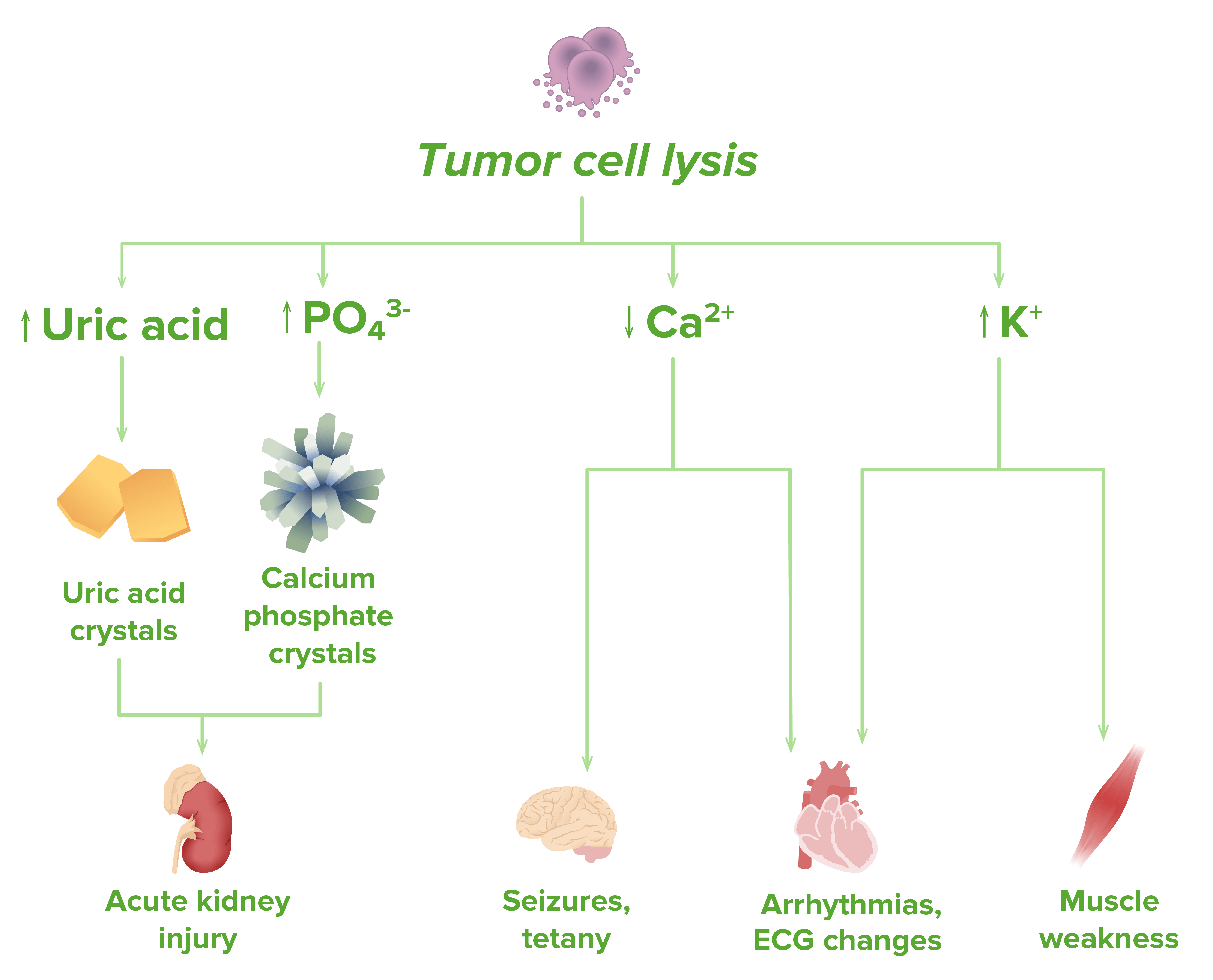
Pathophysiology of tumor lysis syndrome
Image by Lecturio.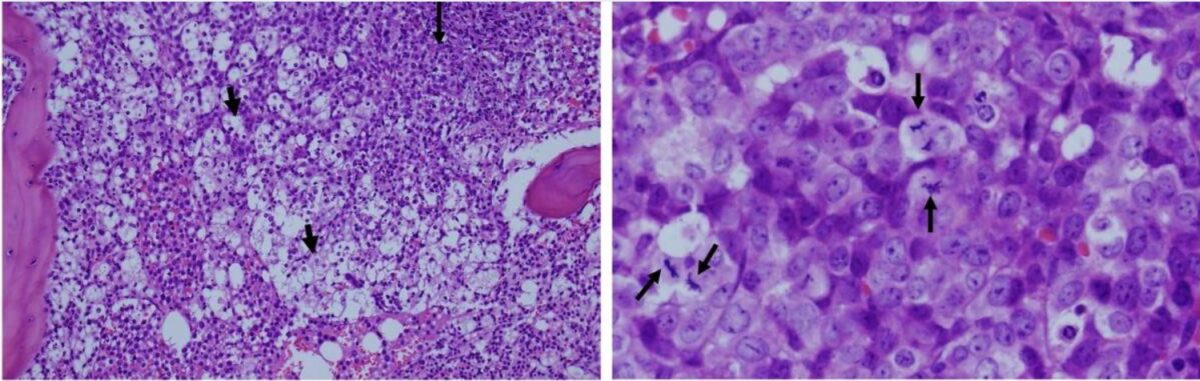
Histological images taken from a patient with leukemia:
Left: The white areas represent extreme apoptosis of leukemic cells. Cell destruction leads to a release of potassium, phosphate, and nucleic acids, which contributes to tumor lysis syndrome.
Right: higher magnification of viable and proliferating leukemic cells
Symptoms typically occur within 72 hours of the initiation of cytotoxic Cytotoxic Parvovirus B19 therapy and are the consequence of hyperkalemia Hyperkalemia Hyperkalemia is defined as a serum potassium (K+) concentration >5.2 mEq/L. Homeostatic mechanisms maintain the serum K+ concentration between 3.5 and 5.2 mEq/L, despite marked variation in dietary intake. Hyperkalemia can be due to a variety of causes, which include transcellular shifts, tissue breakdown, inadequate renal excretion, and drugs. Hyperkalemia, hyperphosphatemia Hyperphosphatemia A condition of abnormally high level of phosphates in the blood, usually significantly above the normal range of 0. 84-1. 58 mmol per liter of serum. Hypocalcemia, hypocalcemia Hypocalcemia Hypocalcemia, a serum calcium < 8.5 mg/dL, can result from various conditions. The causes may include hypoparathyroidism, drugs, disorders leading to vitamin D deficiency, and more. Calcium levels are regulated and affected by different elements such as dietary intake, parathyroid hormone (PTH), vitamin D, pH, and albumin. Presentation can range from an asymptomatic (mild deficiency) to a life-threatening condition (acute, significant deficiency). Hypocalcemia, and hyperuricemia Hyperuricemia Excessive uric acid or urate in blood as defined by its solubility in plasma at 37 degrees c; greater than 0. 42 mmol per liter (7. 0 mg/dl) in men or 0. 36 mmol per liter (6. 0 mg/dl) in women. Gout.
In addition to the clinical signs and symptoms, a laboratory diagnosis can be made with ≥ 2 of the following criteria (in the setting of recent cytotoxic Cytotoxic Parvovirus B19 therapy):
Patients Patients Individuals participating in the health care system for the purpose of receiving therapeutic, diagnostic, or preventive procedures. Clinician–Patient Relationship should also be evaluated for evidence of renal failure Renal failure Conditions in which the kidneys perform below the normal level in the ability to remove wastes, concentrate urine, and maintain electrolyte balance; blood pressure; and calcium metabolism. Renal insufficiency can be classified by the degree of kidney damage (as measured by the level of proteinuria) and reduction in glomerular filtration rate. Crush Syndrome:
The following may be used to manage and prevent tumor Tumor Inflammation lysis syndrome in high-risk patients Patients Individuals participating in the health care system for the purpose of receiving therapeutic, diagnostic, or preventive procedures. Clinician–Patient Relationship: