Acute myeloid leukemia (AML) is a hematologic malignancy Malignancy Hemothorax characterized by the uncontrolled proliferation of myeloid precursor cells. Seen predominantly in older adults, AML includes an accumulation of myeloblasts and a replacement of normal marrow by malignant cells, which leads to impaired hematopoiesis Hematopoiesis The development and formation of various types of blood cells. Hematopoiesis can take place in the bone marrow (medullary) or outside the bone marrow (extramedullary hematopoiesis). Bone Marrow: Composition and Hematopoiesis. Clinical presentation consisting of fatigue Fatigue The state of weariness following a period of exertion, mental or physical, characterized by a decreased capacity for work and reduced efficiency to respond to stimuli. Fibromyalgia, bleeding, fever Fever Fever is defined as a measured body temperature of at least 38°C (100.4°F). Fever is caused by circulating endogenous and/or exogenous pyrogens that increase levels of prostaglandin E2 in the hypothalamus. Fever is commonly associated with chills, rigors, sweating, and flushing of the skin. Fever, and infection is related to anemia Anemia Anemia is a condition in which individuals have low Hb levels, which can arise from various causes. Anemia is accompanied by a reduced number of RBCs and may manifest with fatigue, shortness of breath, pallor, and weakness. Subtypes are classified by the size of RBCs, chronicity, and etiology. Anemia: Overview and Types, thrombocytopenia Thrombocytopenia Thrombocytopenia occurs when the platelet count is < 150,000 per microliter. The normal range for platelets is usually 150,000-450,000/µL of whole blood. Thrombocytopenia can be a result of decreased production, increased destruction, or splenic sequestration of platelets. Patients are often asymptomatic until platelet counts are < 50,000/µL. Thrombocytopenia, and a lack of functional WBCs. The onset of symptoms takes days to weeks. Additional findings in AML may include gingival hypertrophy Hypertrophy General increase in bulk of a part or organ due to cell enlargement and accumulation of fluids and secretions, not due to tumor formation, nor to an increase in the number of cells (hyperplasia). Cellular Adaptation and skin Skin The skin, also referred to as the integumentary system, is the largest organ of the body. The skin is primarily composed of the epidermis (outer layer) and dermis (deep layer). The epidermis is primarily composed of keratinocytes that undergo rapid turnover, while the dermis contains dense layers of connective tissue. Skin: Structure and Functions infiltration (leukemia cutis). Diagnosis is via peripheral blood smear Peripheral Blood Smear Anemia: Overview and Types and bone-marrow biopsy Biopsy Removal and pathologic examination of specimens from the living body. Ewing Sarcoma examination (shows myeloblasts). The precursor cells contain Auer rods. Immunophenotyping Immunophenotyping Process of classifying cells of the immune system based on structural and functional differences. The process is commonly used to analyze and sort T-lymphocytes into subsets based on CD antigens by the technique of flow cytometry. Non-Hodgkin Lymphomas, histochemistry, and genetic analysis all aid in identifying and guiding the treatment of AML. Management is chemotherapy Chemotherapy Osteosarcoma administered in phases (induction, consolidation Consolidation Pulmonary Function Tests, and maintenance) based on subtypes. Prognosis Prognosis A prediction of the probable outcome of a disease based on a individual's condition and the usual course of the disease as seen in similar situations. Non-Hodgkin Lymphomas varies according to the age of onset and type of leukemia.
Last updated: May 17, 2024
Acute myeloid leukemia (AML) is a hematologic malignancy Malignancy Hemothorax characterized by the pathological proliferation of myeloid precursor cells in the bone marrow Bone marrow The soft tissue filling the cavities of bones. Bone marrow exists in two types, yellow and red. Yellow marrow is found in the large cavities of large bones and consists mostly of fat cells and a few primitive blood cells. Red marrow is a hematopoietic tissue and is the site of production of erythrocytes and granular leukocytes. Bone marrow is made up of a framework of connective tissue containing branching fibers with the frame being filled with marrow cells. Bone Marrow: Composition and Hematopoiesis and subsequent displacement Displacement The process by which an emotional or behavioral response that is appropriate for one situation appears in another situation for which it is inappropriate. Defense Mechanisms of other blood cell precursors.
The World Health Organization (WHO) classification system is based on multiple factors, including morphology, genetics Genetics Genetics is the study of genes and their functions and behaviors. Basic Terms of Genetics, and clinical features:
Hematopoiesis Hematopoiesis The development and formation of various types of blood cells. Hematopoiesis can take place in the bone marrow (medullary) or outside the bone marrow (extramedullary hematopoiesis). Bone Marrow: Composition and Hematopoiesis begins with a hematopoietic stem cell, which is prompted to divide and differentiate with appropriate chemical stimuli (hemopoietic growth factors).
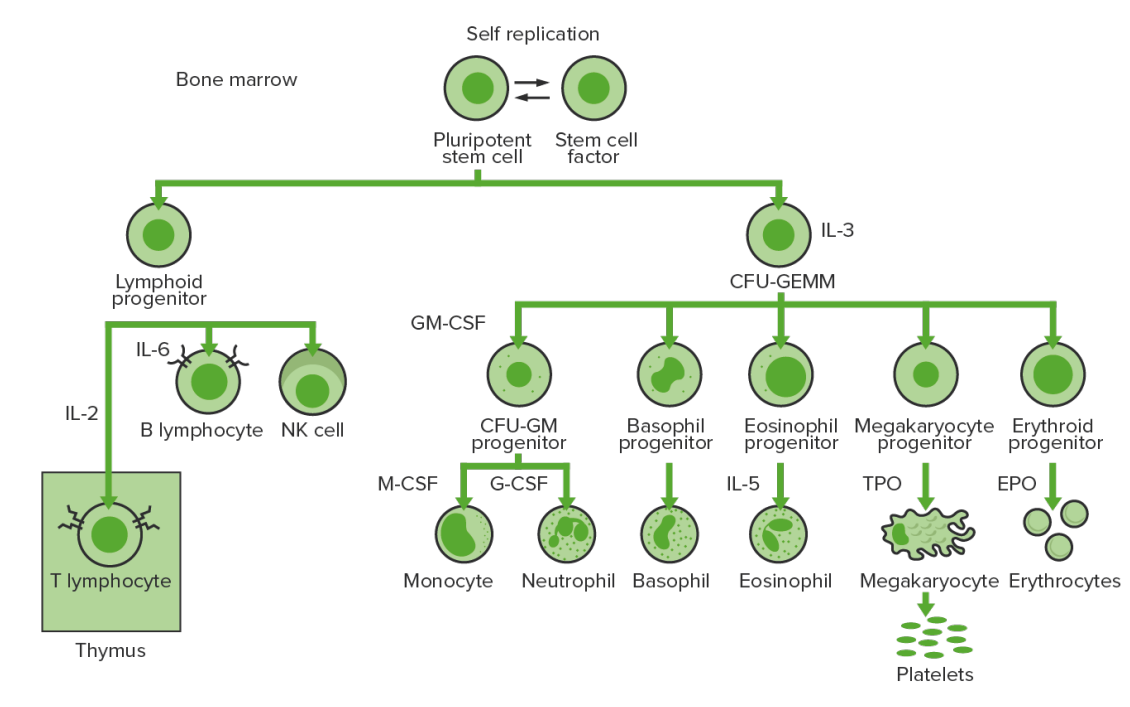
Bone-marrow hematopoiesis: proliferation and differentiation of the formed elements of blood.
CFU-GEMM: colony-forming unit–granulocyte, erythrocyte, monocyte, megakaryocyte
CFU-GM: colony-forming unit–granulocyte-macrophage
GM-CSF: granulocyte-macrophage colony-stimulating factor
M-CSF: macrophage colony-stimulating factor
G-CSF: granulocyte colony-stimulating factor
NK: natural killer
TPO: thrombopoietin
| Features | ALL | AML |
|---|---|---|
| Population | More common in children | More common in adults |
| Common characteristics |
|
|
| Clinical findings |
|
|
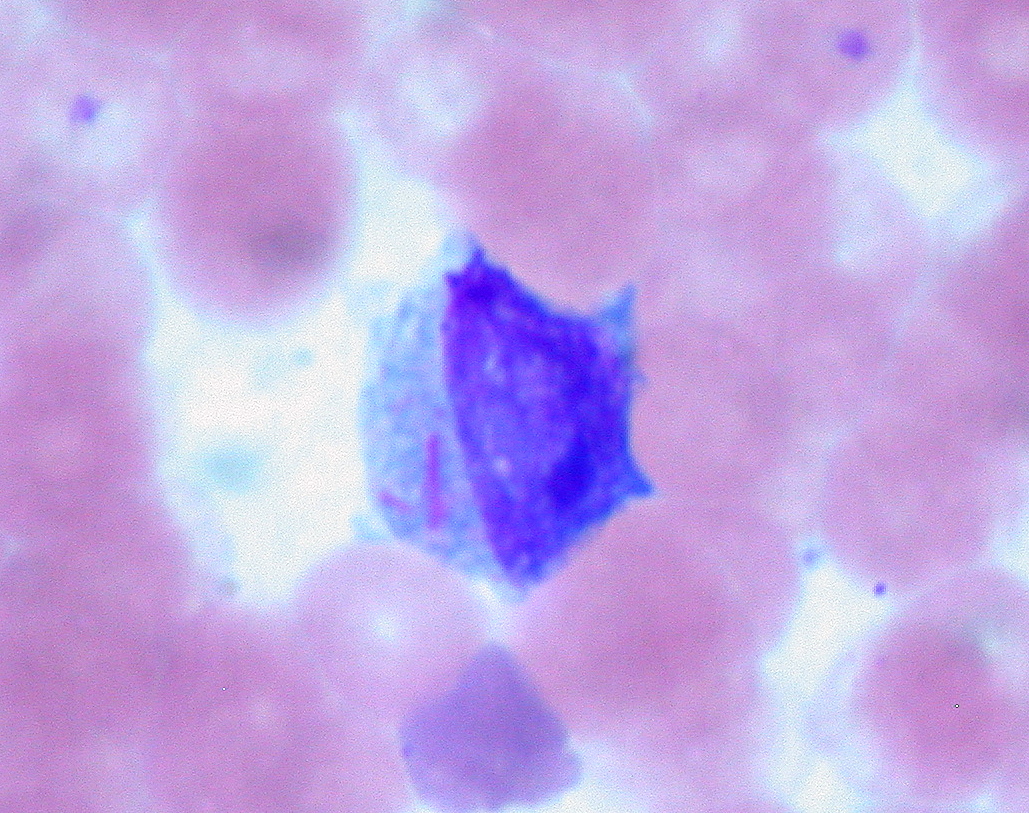
Acute myeloid leukemia (AML): bone-marrow findings of Auer rods (pink, needle-like structures in the cytoplasm) in a myeloblast
Image: “Auer Rods in Leukemic Blast” by Ed Uthman. License: CC BY 2.0| Features | ALL | AML |
|---|---|---|
| Laboratory findings | Anemia Anemia Anemia is a condition in which individuals have low Hb levels, which can arise from various causes. Anemia is accompanied by a reduced number of RBCs and may manifest with fatigue, shortness of breath, pallor, and weakness. Subtypes are classified by the size of RBCs, chronicity, and etiology. Anemia: Overview and Types, thrombocytopenia Thrombocytopenia Thrombocytopenia occurs when the platelet count is < 150,000 per microliter. The normal range for platelets is usually 150,000-450,000/µL of whole blood. Thrombocytopenia can be a result of decreased production, increased destruction, or splenic sequestration of platelets. Patients are often asymptomatic until platelet counts are < 50,000/µL. Thrombocytopenia, varying WBCs | |
| Peripheral smear or bone-marrow examination (morphology) |
Lymphoblasts
Lymphoblasts
Lymphocytosis:
|
Myeloblasts:
|
| Cytochemistry |
|
|
| Immunophenotyping Immunophenotyping Process of classifying cells of the immune system based on structural and functional differences. The process is commonly used to analyze and sort T-lymphocytes into subsets based on CD antigens by the technique of flow cytometry. Non-Hodgkin Lymphomas |
|
CD13, CD33, CD117, HLA-DR |
Chemotherapy Chemotherapy Osteosarcoma requires pretreatment evaluation, including patient goals and preferences, comorbidities Comorbidities The presence of co-existing or additional diseases with reference to an initial diagnosis or with reference to the index condition that is the subject of study. Comorbidity may affect the ability of affected individuals to function and also their survival; it may be used as a prognostic indicator for length of hospital stay, cost factors, and outcome or survival. St. Louis Encephalitis Virus, physical functioning, and prognostic factors related to AML type.
| Management | ALL | AML |
|---|---|---|
| Induction |
|
|
| Consolidation Consolidation Pulmonary Function Tests | Options:
|
Additional chemotherapy Chemotherapy Osteosarcoma ( cytarabine Cytarabine A pyrimidine nucleoside analog that is used mainly in the treatment of leukemia, especially acute non-lymphoblastic leukemia. Cytarabine is an antimetabolite antineoplastic agent that inhibits the synthesis of DNA. Its actions are specific for the s phase of the cell cycle. It also has antiviral and immunosuppressant properties. Antimetabolite Chemotherapy) |
| Maintenance |
|
Non-myelosuppressive chemotherapy Chemotherapy Osteosarcoma and/or a targeted agent |
| Additional treatment |
|
Acute promyelocytic leukemia:
|
| Hematopoietic cell transplantation | For those with a poor prognosis Prognosis A prediction of the probable outcome of a disease based on a individual’s condition and the usual course of the disease as seen in similar situations. Non-Hodgkin Lymphomas | |
| Prognosis Prognosis A prediction of the probable outcome of a disease based on a individual’s condition and the usual course of the disease as seen in similar situations. Non-Hodgkin Lymphomas |
|
|