Microtubule and topoisomerase inhibitors target cellular structures and processes to inhibit cancer cell proliferation. Microtubule inhibitors act on the cytoskeleton Cytoskeleton The network of filaments, tubules, and interconnecting filamentous bridges which give shape, structure, and organization to the cytoplasm. The Cell: Cytosol and Cytoskeleton, while topoisomerase inhibitors act on an enzyme that is important in DNA replication DNA replication The entire DNA of a cell is replicated during the S (synthesis) phase of the cell cycle. The principle of replication is based on complementary nucleotide base pairing: adenine forms hydrogen bonds with thymine (or uracil in RNA) and guanine forms hydrogen bonds with cytosine. DNA Replication and transcription Transcription Transcription of genetic information is the first step in gene expression. Transcription is the process by which DNA is used as a template to make mRNA. This process is divided into 3 stages: initiation, elongation, and termination. Stages of Transcription. The microtubule system, along with microfilaments Microfilaments Fibers composed of microfilament proteins, which are predominately actin. They are the smallest of the cytoskeletal filaments. The Cell: Cytosol and Cytoskeleton and intermediate filaments Intermediate filaments Cytoplasmic filaments intermediate in diameter (about 10 nanometers) between the microfilaments and the microtubules. They may be composed of any of a number of different proteins and form a ring around the cell nucleus. The Cell: Cytosol and Cytoskeleton, form the cellular cytoskeleton Cytoskeleton The network of filaments, tubules, and interconnecting filamentous bridges which give shape, structure, and organization to the cytoplasm. The Cell: Cytosol and Cytoskeleton. These components are essential for cell division Cell Division A type of cell nucleus division by means of which the two daughter nuclei normally receive identical complements of the number of chromosomes of the somatic cells of the species. Cell Cycle, movement, and signaling. Taxanes and vinca alkaloids interfere with microtubule function, and thus in effect, inhibit mitosis Mitosis A type of cell nucleus division by means of which the two daughter nuclei normally receive identical complements of the number of chromosomes of the somatic cells of the species. Cell Cycle. Topoisomerase assists DNA replication DNA replication The entire DNA of a cell is replicated during the S (synthesis) phase of the cell cycle. The principle of replication is based on complementary nucleotide base pairing: adenine forms hydrogen bonds with thymine (or uracil in RNA) and guanine forms hydrogen bonds with cytosine. DNA Replication by creating double- and single-stranded breaks to relieve supercoils. Inhibiting the enzyme causes termination of DNA replication DNA replication The entire DNA of a cell is replicated during the S (synthesis) phase of the cell cycle. The principle of replication is based on complementary nucleotide base pairing: adenine forms hydrogen bonds with thymine (or uracil in RNA) and guanine forms hydrogen bonds with cytosine. DNA Replication and DNA damage DNA Damage Injuries to DNA that introduce deviations from its normal, intact structure and which may, if left unrepaired, result in a mutation or a block of DNA replication. These deviations may be caused by physical or chemical agents and occur by natural or unnatural, introduced circumstances. They include the introduction of illegitimate bases during replication or by deamination or other modification of bases; the loss of a base from the DNA backbone leaving an abasic site; single-strand breaks; double strand breaks; and intrastrand (pyrimidine dimers) or interstrand crosslinking. Damage can often be repaired (DNA repair). If the damage is extensive, it can induce apoptosis. DNA Repair Mechanisms. There are multiple chemotherapeutic agents in each class that commonly produce myelosuppression Myelosuppression Oxazolidinones as an adverse effect.
Last updated: Apr 13, 2025
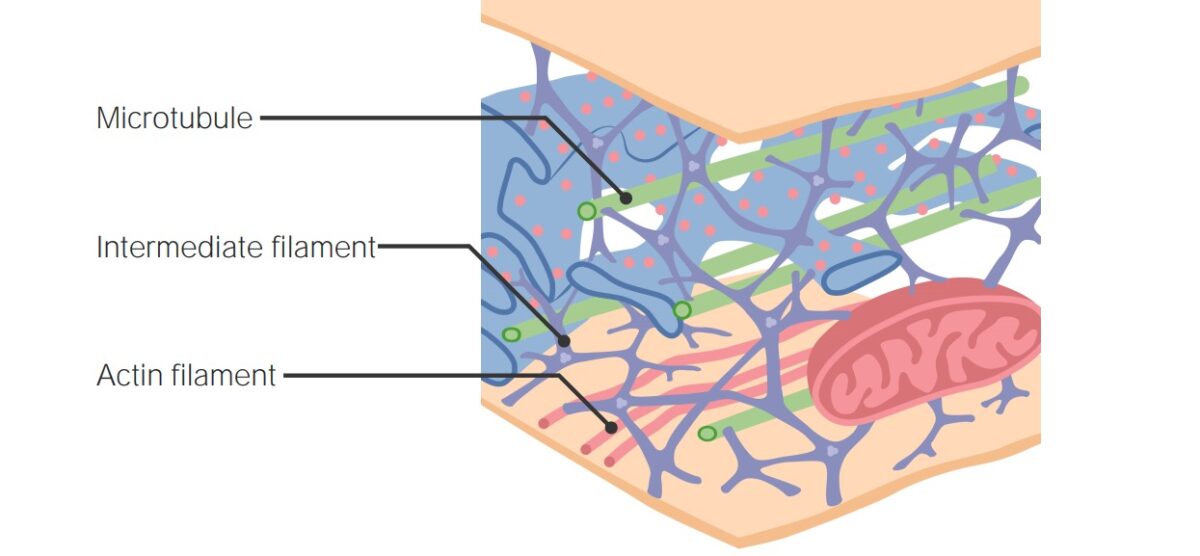
Filaments of the cytoskeleton
Image by Lecturio.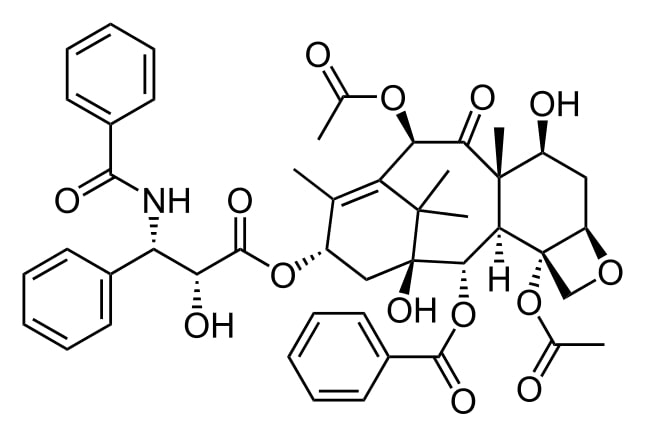
Structure of paclitaxel
Image: “Taxol” by Calvero. License: Public Domain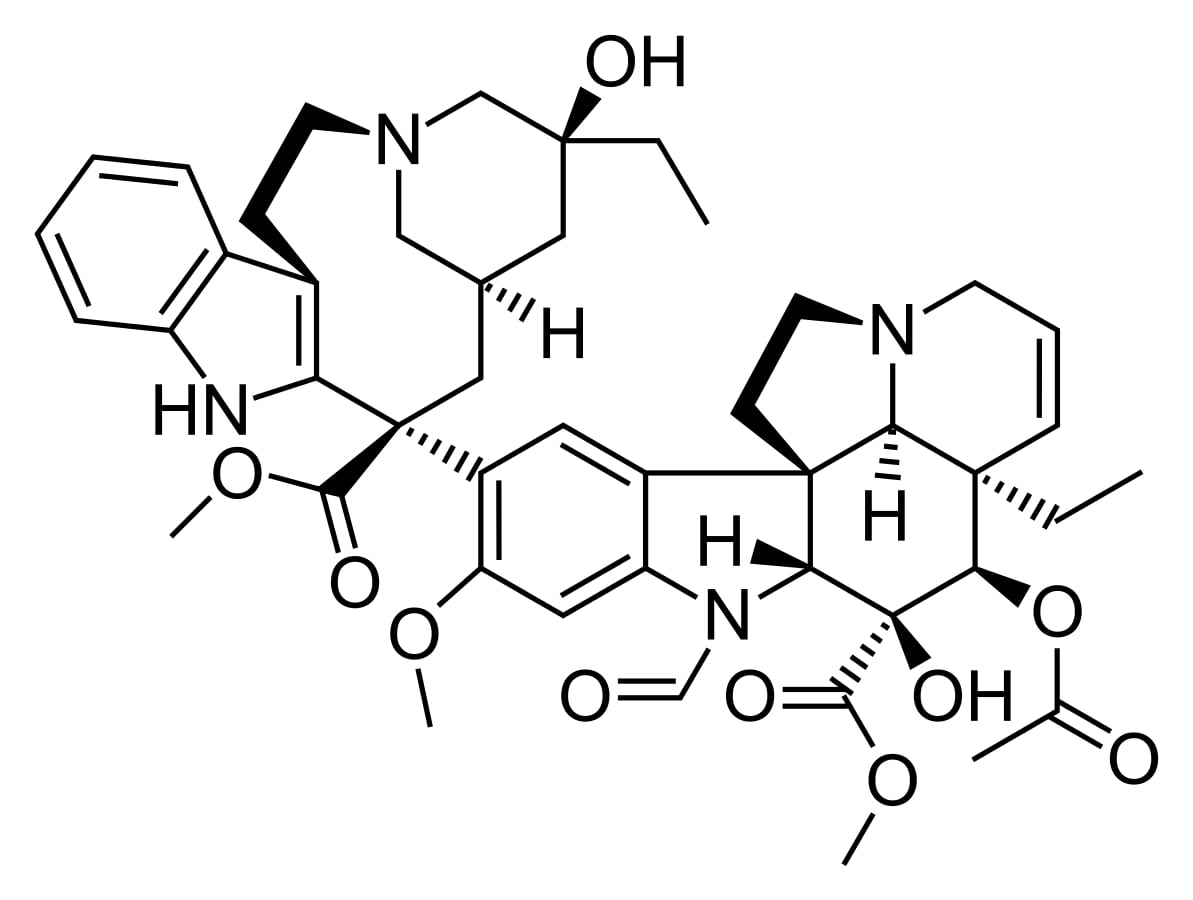
Structure of vincristine
Image: “Vincristine” by Fvasconcellos. License: Public Domain
Structure of irinotecan
Image: “Irinotecan” by Fvasconcellos. License: Public Domain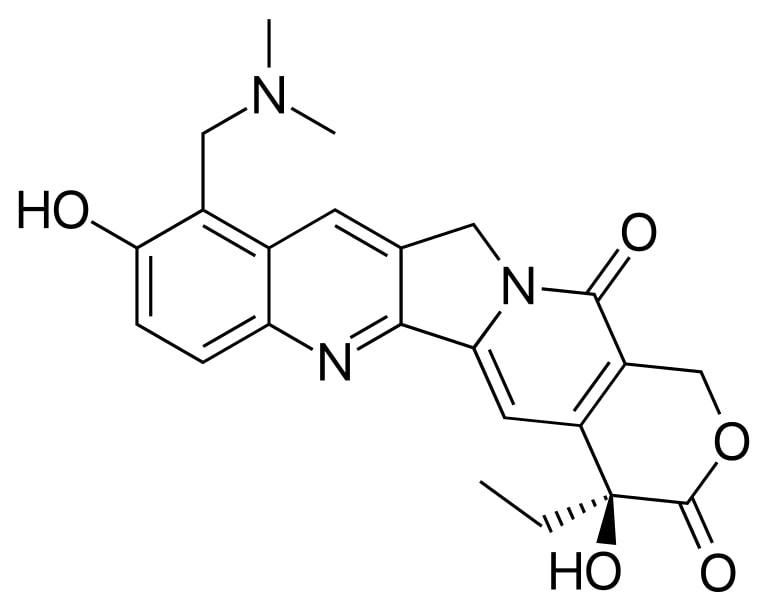
Structure of topotecan
Image: “Topotecan” by Fvasconcellos. License: Public Domain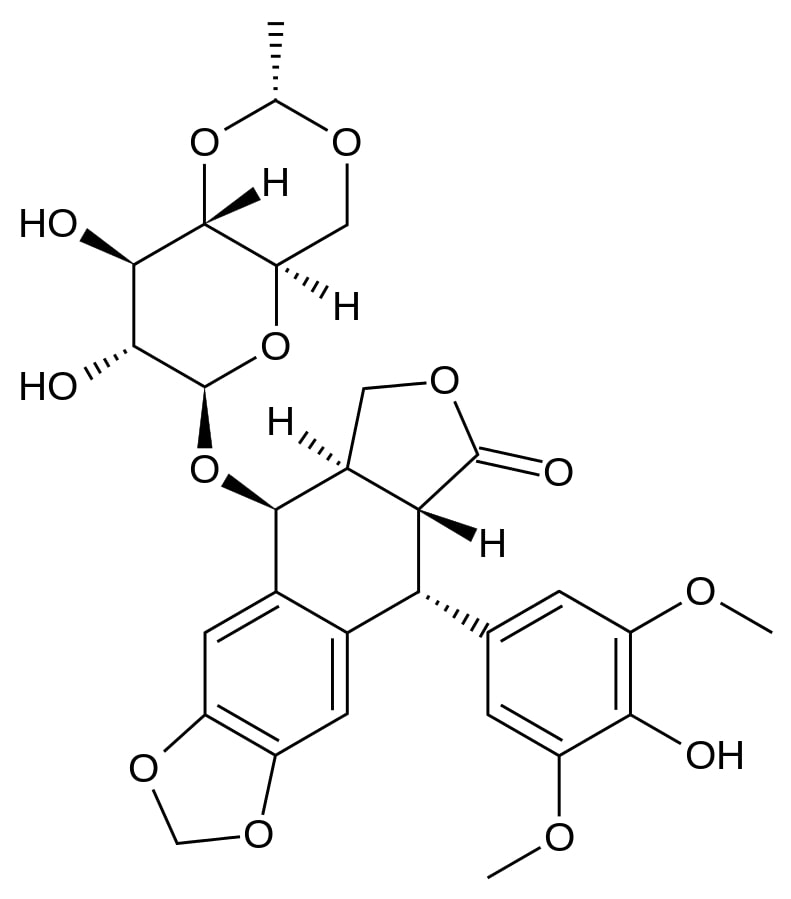
Structure of etoposide
Image: “Etoposide” by Fvasconcellos. License: Public Domain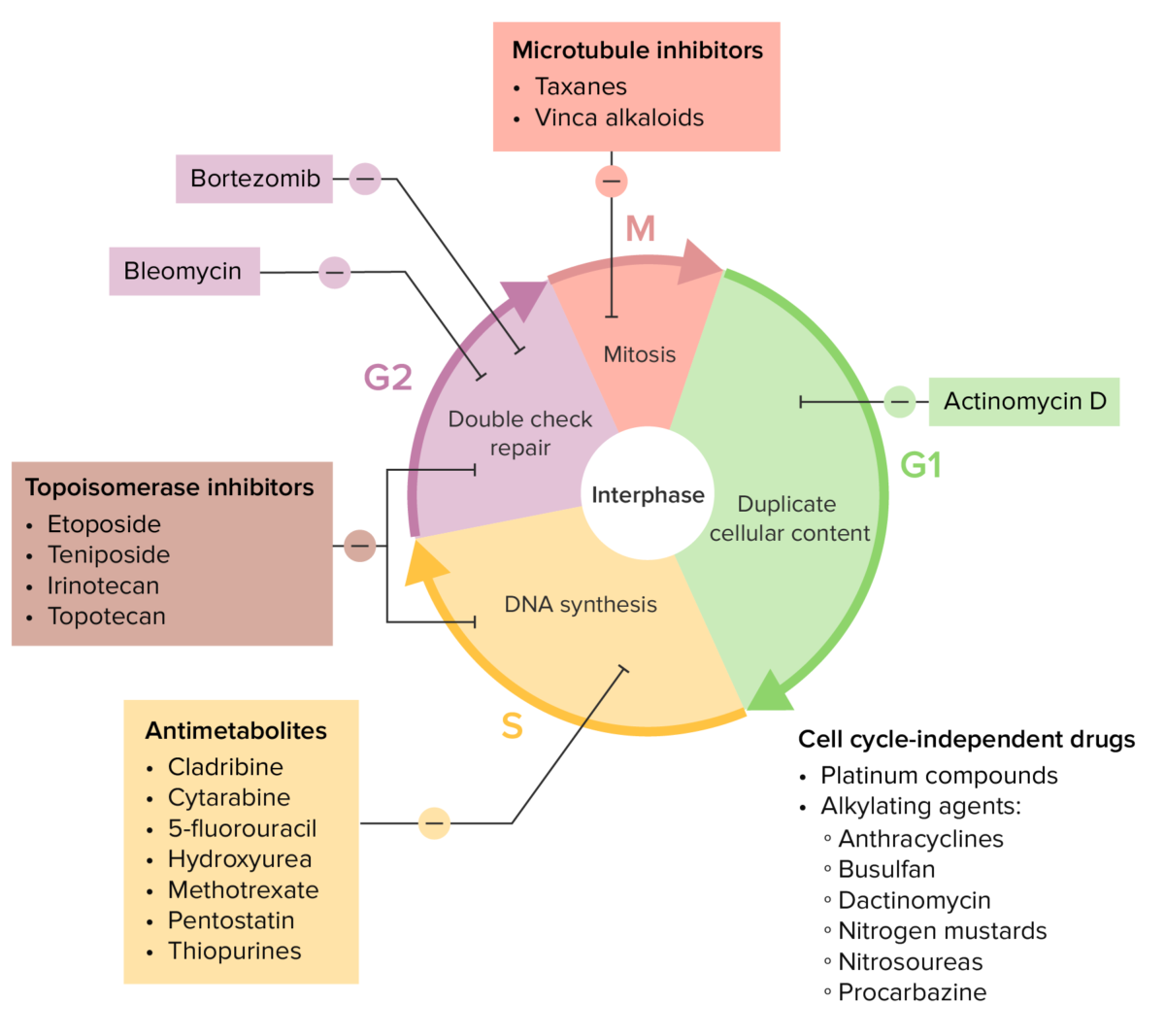
Various chemotherapy drugs and their effects on the cell cycle
Image by Lecturio.| Drug class | Mechanism |
|---|---|
Antitumor antibiotics
Antitumor Antibiotics
Antitumor antibiotics, also known as antineoplastic antibiotics, are the product of soil microbes, Streptomyces bacteria. The commonly used types of antitumor antibiotics (bleomycin, dactinomycin, and anthracyclines) have a wide spectrum of activity against hematologic malignancies and solid tumors.
Antitumor Antibiotics:
|
Intercalate between bases Bases Usually a hydroxide of lithium, sodium, potassium, rubidium or cesium, but also the carbonates of these metals, ammonia, and the amines. Acid-Base Balance, leading to blockage of DNA DNA A deoxyribonucleotide polymer that is the primary genetic material of all cells. Eukaryotic and prokaryotic organisms normally contain DNA in a double-stranded state, yet several important biological processes transiently involve single-stranded regions. DNA, which consists of a polysugar-phosphate backbone possessing projections of purines (adenine and guanine) and pyrimidines (thymine and cytosine), forms a double helix that is held together by hydrogen bonds between these purines and pyrimidines (adenine to thymine and guanine to cytosine). DNA Types and Structure or RNA RNA A polynucleotide consisting essentially of chains with a repeating backbone of phosphate and ribose units to which nitrogenous bases are attached. RNA is unique among biological macromolecules in that it can encode genetic information, serve as an abundant structural component of cells, and also possesses catalytic activity. RNA Types and Structure synthesis Synthesis Polymerase Chain Reaction (PCR) and prevention of DNA replication DNA replication The entire DNA of a cell is replicated during the S (synthesis) phase of the cell cycle. The principle of replication is based on complementary nucleotide base pairing: adenine forms hydrogen bonds with thymine (or uracil in RNA) and guanine forms hydrogen bonds with cytosine. DNA Replication |
| Anthracyclines Anthracyclines Organic compounds that have a tetrahydronaphthacenedione ring structure attached by a glycosidic linkage to the amino sugar daunosamine. Antitumor Antibiotics |
|
| Alkylating agents |
|
| Drug class | Cell cycle Cell cycle The phases of the cell cycle include interphase (G1, S, and G2) and mitosis (prophase, metaphase, anaphase, and telophase). The cell’s progression through these phases is punctuated by checkpoints regulated by cyclins, cyclin-dependent kinases, tumor suppressors, and their antagonists. Cell Cycle phase affected | Mechanism of action |
|---|---|---|
| Antifolates Antifolates Inhibitors of the enzyme, dihydrofolate reductase, which converts dihydrofolate (fh2) to tetrahydrofolate (fh4). They are frequently used in cancer chemotherapy. Antimetabolite Chemotherapy | Cell cycle Cell cycle The phases of the cell cycle include interphase (G1, S, and G2) and mitosis (prophase, metaphase, anaphase, and telophase). The cell’s progression through these phases is punctuated by checkpoints regulated by cyclins, cyclin-dependent kinases, tumor suppressors, and their antagonists. Cell Cycle arrest at S phase S Phase Phase of the cell cycle following g1 and preceding g2 when the entire DNA content of the nucleus is replicated. It is achieved by bidirectional replication at multiple sites along each chromosome. Cell Cycle | Inhibit:
|
| Bleomycin Bleomycin A complex of related glycopeptide antibiotics from streptomyces verticillus consisting of bleomycin a2 and b2. It inhibits DNA metabolism and is used as an antineoplastic, especially for solid tumors. Antitumor Antibiotics | Cell-cycle arrest at G2 phase G2 Phase The period of the cell cycle following DNA synthesis (S phase) and preceding m phase (cell division phase). The chromosomes are tetraploid in this point. Cell Cycle | Binds DNA DNA A deoxyribonucleotide polymer that is the primary genetic material of all cells. Eukaryotic and prokaryotic organisms normally contain DNA in a double-stranded state, yet several important biological processes transiently involve single-stranded regions. DNA, which consists of a polysugar-phosphate backbone possessing projections of purines (adenine and guanine) and pyrimidines (thymine and cytosine), forms a double helix that is held together by hydrogen bonds between these purines and pyrimidines (adenine to thymine and guanine to cytosine). DNA Types and Structure, leading to single- and double-stranded breaks |
| Fluoropyrimidines | Cell cycle Cell cycle The phases of the cell cycle include interphase (G1, S, and G2) and mitosis (prophase, metaphase, anaphase, and telophase). The cell’s progression through these phases is punctuated by checkpoints regulated by cyclins, cyclin-dependent kinases, tumor suppressors, and their antagonists. Cell Cycle arrest at S phase S Phase Phase of the cell cycle following g1 and preceding g2 when the entire DNA content of the nucleus is replicated. It is achieved by bidirectional replication at multiple sites along each chromosome. Cell Cycle | Inhibit thymidylate synthase Thymidylate synthase An enzyme of the transferase class that catalyzes the reaction 5, 10-methylenetetrahydrofolate and dump to dihydrofolate and dtmp in the synthesis of thymidine triphosphate. Purine and Pyrimidine Metabolism |
| Deoxycytidine analogs | Cell cycle Cell cycle The phases of the cell cycle include interphase (G1, S, and G2) and mitosis (prophase, metaphase, anaphase, and telophase). The cell’s progression through these phases is punctuated by checkpoints regulated by cyclins, cyclin-dependent kinases, tumor suppressors, and their antagonists. Cell Cycle arrest at S phase S Phase Phase of the cell cycle following g1 and preceding g2 when the entire DNA content of the nucleus is replicated. It is achieved by bidirectional replication at multiple sites along each chromosome. Cell Cycle | Inhibit:
|
| Purine analogs Purine Analogs Antimetabolite Chemotherapy | Cell cycle Cell cycle The phases of the cell cycle include interphase (G1, S, and G2) and mitosis (prophase, metaphase, anaphase, and telophase). The cell’s progression through these phases is punctuated by checkpoints regulated by cyclins, cyclin-dependent kinases, tumor suppressors, and their antagonists. Cell Cycle arrest at S phase S Phase Phase of the cell cycle following g1 and preceding g2 when the entire DNA content of the nucleus is replicated. It is achieved by bidirectional replication at multiple sites along each chromosome. Cell Cycle | Inhibition of de novo purine synthesis Synthesis Polymerase Chain Reaction (PCR) |
| Topoisomerase II Topoisomerase II DNA topoisomerases that catalyze ATP-dependent breakage of both strands of DNA, passage of the unbroken strands through the breaks, and rejoining of the broken strands. These enzymes bring about relaxation of the supercoiled DNA and resolution of a knotted circular DNA duplex. Fluoroquinolones inhibitors | Cell-cycle arrest at S and G2 phases | Inhibit topoisomerase II Topoisomerase II DNA topoisomerases that catalyze ATP-dependent breakage of both strands of DNA, passage of the unbroken strands through the breaks, and rejoining of the broken strands. These enzymes bring about relaxation of the supercoiled DNA and resolution of a knotted circular DNA duplex. Fluoroquinolones |
| Taxanes | Cell-cycle arrest at metaphase Metaphase The phase of cell nucleus division following prometaphase, in which the chromosomes line up across the equatorial plane of the spindle apparatus prior to separation. Cell Cycle of the M phase | Hyperstabilization of microtubules Microtubules Slender, cylindrical filaments found in the cytoskeleton of plant and animal cells. They are composed of the protein tubulin and are influenced by tubulin modulators. The Cell: Cytosol and Cytoskeleton |
| Vinca alkaloids | Cell-cycle arrest during metaphase Metaphase The phase of cell nucleus division following prometaphase, in which the chromosomes line up across the equatorial plane of the spindle apparatus prior to separation. Cell Cycle of the M phase | Binds to beta-tubulin and prevents microtubule polymerization |