The gonadal hormones Hormones Hormones are messenger molecules that are synthesized in one part of the body and move through the bloodstream to exert specific regulatory effects on another part of the body. Hormones play critical roles in coordinating cellular activities throughout the body in response to the constant changes in both the internal and external environments. Hormones: Overview and Types are produced by the human gonads Gonads The gamete-producing glands, ovary or testis. Hormones: Overview and Types: the testes and the ovaries Ovaries Ovaries are the paired gonads of the female reproductive system that contain haploid gametes known as oocytes. The ovaries are located intraperitoneally in the pelvis, just posterior to the broad ligament, and are connected to the pelvic sidewall and to the uterus by ligaments. These organs function to secrete hormones (estrogen and progesterone) and to produce the female germ cells (oocytes). Ovaries: Anatomy. The primary hormones Hormones Hormones are messenger molecules that are synthesized in one part of the body and move through the bloodstream to exert specific regulatory effects on another part of the body. Hormones play critical roles in coordinating cellular activities throughout the body in response to the constant changes in both the internal and external environments. Hormones: Overview and Types produced by these organs include androgens Androgens Androgens are naturally occurring steroid hormones responsible for development and maintenance of the male sex characteristics, including penile, scrotal, and clitoral growth, development of sexual hair, deepening of the voice, and musculoskeletal growth. Androgens and Antiandrogens, estrogens, and progestins Progestins Compounds that interact with progesterone receptors in target tissues to bring about the effects similar to those of progesterone. Primary actions of progestins, including natural and synthetic steroids, are on the uterus and the mammary gland in preparation for and in maintenance of pregnancy. Hormonal Contraceptives. Testosterone Testosterone A potent androgenic steroid and major product secreted by the leydig cells of the testis. Its production is stimulated by luteinizing hormone from the pituitary gland. In turn, testosterone exerts feedback control of the pituitary LH and FSH secretion. Depending on the tissues, testosterone can be further converted to dihydrotestosterone or estradiol. Androgens and Antiandrogens is the primary androgen, and it plays a critical role in the development of the primary and secondary male sex Sex The totality of characteristics of reproductive structure, functions, phenotype, and genotype, differentiating the male from the female organism. Gender Dysphoria characteristics, as well as in spermatogenesis Spermatogenesis The process of germ cell development in the male from the primordial germ cells, through spermatogonia; spermatocytes; spermatids; to the mature haploid spermatozoa. Gametogenesis. Estradiol Estradiol The 17-beta-isomer of estradiol, an aromatized C18 steroid with hydroxyl group at 3-beta- and 17-beta-position. Estradiol-17-beta is the most potent form of mammalian estrogenic steroids. Noncontraceptive Estrogen and Progestins and progesterone are the primary female hormones Hormones Hormones are messenger molecules that are synthesized in one part of the body and move through the bloodstream to exert specific regulatory effects on another part of the body. Hormones play critical roles in coordinating cellular activities throughout the body in response to the constant changes in both the internal and external environments. Hormones: Overview and Types, which are responsible for egg development, the menstrual cycle Menstrual cycle The menstrual cycle is the cyclic pattern of hormonal and tissular activity that prepares a suitable uterine environment for the fertilization and implantation of an ovum. The menstrual cycle involves both an endometrial and ovarian cycle that are dependent on one another for proper functioning. There are 2 phases of the ovarian cycle and 3 phases of the endometrial cycle. Menstrual Cycle, and breast development. The gonadal hormones Hormones Hormones are messenger molecules that are synthesized in one part of the body and move through the bloodstream to exert specific regulatory effects on another part of the body. Hormones play critical roles in coordinating cellular activities throughout the body in response to the constant changes in both the internal and external environments. Hormones: Overview and Types are part of the hypothalamic-pituitary-gonadal (HPG) axis and are regulated by the pituitary Pituitary A small, unpaired gland situated in the sella turcica. It is connected to the hypothalamus by a short stalk which is called the infundibulum. Hormones: Overview and Types hormones Hormones Hormones are messenger molecules that are synthesized in one part of the body and move through the bloodstream to exert specific regulatory effects on another part of the body. Hormones play critical roles in coordinating cellular activities throughout the body in response to the constant changes in both the internal and external environments. Hormones: Overview and Types follicle-stimulating hormone ( FSH FSH A major gonadotropin secreted by the adenohypophysis. Follicle-stimulating hormone stimulates gametogenesis and the supporting cells such as the ovarian granulosa cells, the testicular sertoli cells, and leydig cells. Fsh consists of two noncovalently linked subunits, alpha and beta. Within a species, the alpha subunit is common in the three pituitary glycoprotein hormones (TSH, LH, and FSH), but the beta subunit is unique and confers its biological specificity. Menstrual Cycle) and luteinizing hormone ( LH LH A major gonadotropin secreted by the adenohypophysis. Luteinizing hormone regulates steroid production by the interstitial cells of the testis and the ovary. The preovulatory luteinizing hormone surge in females induces ovulation, and subsequent luteinization of the follicle. Luteinizing hormone consists of two noncovalently linked subunits, alpha and beta. Within a species, the alpha subunit is common in the three pituitary glycoprotein hormones (TSH, LH, and FSH), but the beta subunit is unique and confers its biological specificity. Menstrual Cycle). In turn, FSH FSH A major gonadotropin secreted by the adenohypophysis. Follicle-stimulating hormone stimulates gametogenesis and the supporting cells such as the ovarian granulosa cells, the testicular sertoli cells, and leydig cells. Fsh consists of two noncovalently linked subunits, alpha and beta. Within a species, the alpha subunit is common in the three pituitary glycoprotein hormones (TSH, LH, and FSH), but the beta subunit is unique and confers its biological specificity. Menstrual Cycle and LH LH A major gonadotropin secreted by the adenohypophysis. Luteinizing hormone regulates steroid production by the interstitial cells of the testis and the ovary. The preovulatory luteinizing hormone surge in females induces ovulation, and subsequent luteinization of the follicle. Luteinizing hormone consists of two noncovalently linked subunits, alpha and beta. Within a species, the alpha subunit is common in the three pituitary glycoprotein hormones (TSH, LH, and FSH), but the beta subunit is unique and confers its biological specificity. Menstrual Cycle are both regulated by gonadotropin-releasing hormone Gonadotropin-releasing hormone A decapeptide that stimulates the synthesis and secretion of both pituitary gonadotropins, luteinizing hormone and follicle stimulating hormone. Gnrh is produced by neurons in the septum preoptic area of the hypothalamus and released into the pituitary portal blood, leading to stimulation of gonadotrophs in the anterior pituitary gland. Puberty (GnRH) secreted from the hypothalamus Hypothalamus The hypothalamus is a collection of various nuclei within the diencephalon in the center of the brain. The hypothalamus plays a vital role in endocrine regulation as the primary regulator of the pituitary gland, and it is the major point of integration between the central nervous and endocrine systems. Hypothalamus.
Last updated: May 17, 2024
Gonadal hormones Hormones Hormones are messenger molecules that are synthesized in one part of the body and move through the bloodstream to exert specific regulatory effects on another part of the body. Hormones play critical roles in coordinating cellular activities throughout the body in response to the constant changes in both the internal and external environments. Hormones: Overview and Types:
The gonadal hormones Hormones Hormones are messenger molecules that are synthesized in one part of the body and move through the bloodstream to exert specific regulatory effects on another part of the body. Hormones play critical roles in coordinating cellular activities throughout the body in response to the constant changes in both the internal and external environments. Hormones: Overview and Types are produced by the human gonads Gonads The gamete-producing glands, ovary or testis. Hormones: Overview and Types: the testes and ovaries Ovaries Ovaries are the paired gonads of the female reproductive system that contain haploid gametes known as oocytes. The ovaries are located intraperitoneally in the pelvis, just posterior to the broad ligament, and are connected to the pelvic sidewall and to the uterus by ligaments. These organs function to secrete hormones (estrogen and progesterone) and to produce the female germ cells (oocytes). Ovaries: Anatomy. These hormones Hormones Hormones are messenger molecules that are synthesized in one part of the body and move through the bloodstream to exert specific regulatory effects on another part of the body. Hormones play critical roles in coordinating cellular activities throughout the body in response to the constant changes in both the internal and external environments. Hormones: Overview and Types include:
Gendered terminology:
Recognizing the complexity and sensitive nature around gendered terminology, for the purposes of this document:
Hormones Hormones Hormones are messenger molecules that are synthesized in one part of the body and move through the bloodstream to exert specific regulatory effects on another part of the body. Hormones play critical roles in coordinating cellular activities throughout the body in response to the constant changes in both the internal and external environments. Hormones: Overview and Types secreted by the gonads Gonads The gamete-producing glands, ovary or testis. Hormones: Overview and Types are stimulated by, and help to regulate, the hypothalamic-pituitary-testicular (HPT) axis in males and the hypothalamic-pituitary-ovarian (HPO) axis in females.
All of the gonadal hormones Hormones Hormones are messenger molecules that are synthesized in one part of the body and move through the bloodstream to exert specific regulatory effects on another part of the body. Hormones play critical roles in coordinating cellular activities throughout the body in response to the constant changes in both the internal and external environments. Hormones: Overview and Types are steroids Steroids A group of polycyclic compounds closely related biochemically to terpenes. They include cholesterol, numerous hormones, precursors of certain vitamins, bile acids, alcohols (sterols), and certain natural drugs and poisons. Steroids have a common nucleus, a fused, reduced 17-carbon atom ring system, cyclopentanoperhydrophenanthrene. Most steroids also have two methyl groups and an aliphatic side-chain attached to the nucleus. Benign Liver Tumors, produced from cholesterol Cholesterol The principal sterol of all higher animals, distributed in body tissues, especially the brain and spinal cord, and in animal fats and oils. Cholesterol Metabolism via a series of enzymatic steps. The steps in the sex Sex The totality of characteristics of reproductive structure, functions, phenotype, and genotype, differentiating the male from the female organism. Gender Dysphoria hormone metabolic pathway include:
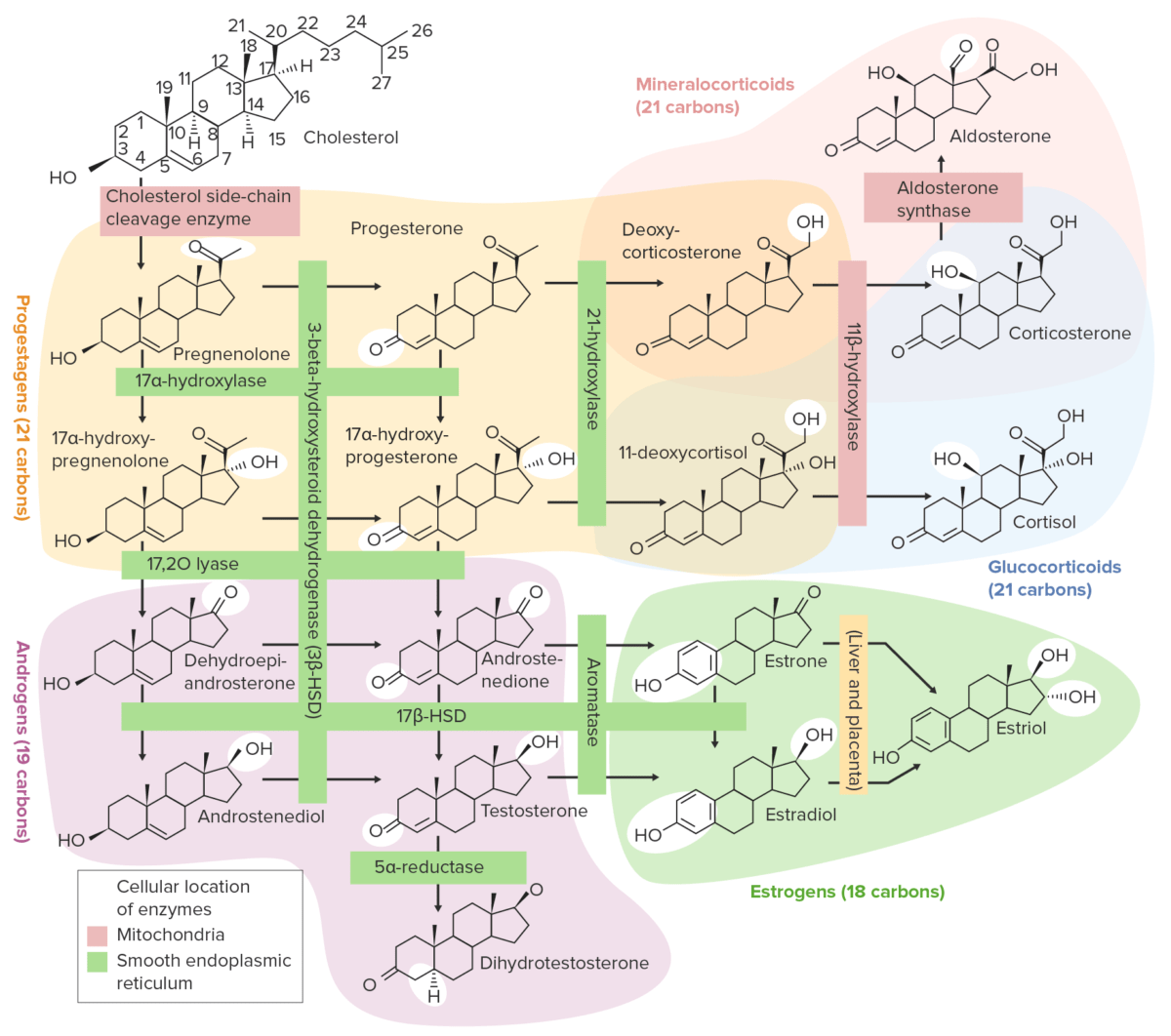
Overview of the steroidogenesis pathways
HSD: hydroxysteroid dehydrogenase
Derived from cholesterol Cholesterol The principal sterol of all higher animals, distributed in body tissues, especially the brain and spinal cord, and in animal fats and oils. Cholesterol Metabolism, the gonadal hormones Hormones Hormones are messenger molecules that are synthesized in one part of the body and move through the bloodstream to exert specific regulatory effects on another part of the body. Hormones play critical roles in coordinating cellular activities throughout the body in response to the constant changes in both the internal and external environments. Hormones: Overview and Types are lipophilic, so they must be protein-bound to travel in the blood. In general, they are bound to:

Diagram depicting the effect of gonadal hormones on cells using testosterone as an example
DHT: dihydrotestosterone
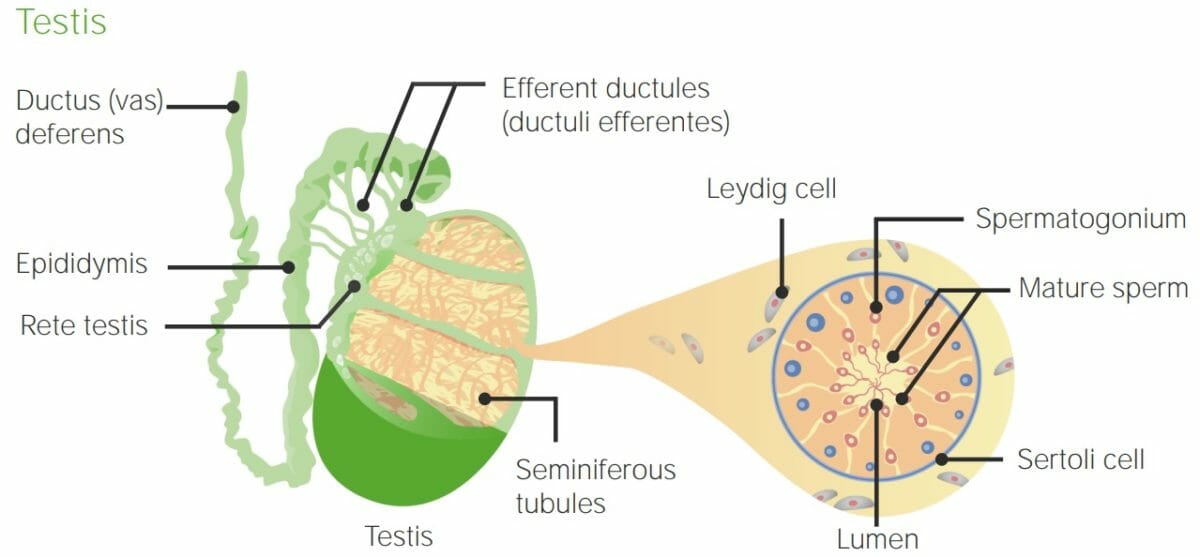
Anatomy of the testis and seminiferous tubules: Note the tubules are created by Sertoli cells and surrounded by Leydig cells. Spermatogenesis takes place within the seminiferous tubules.
Image by Lecturio.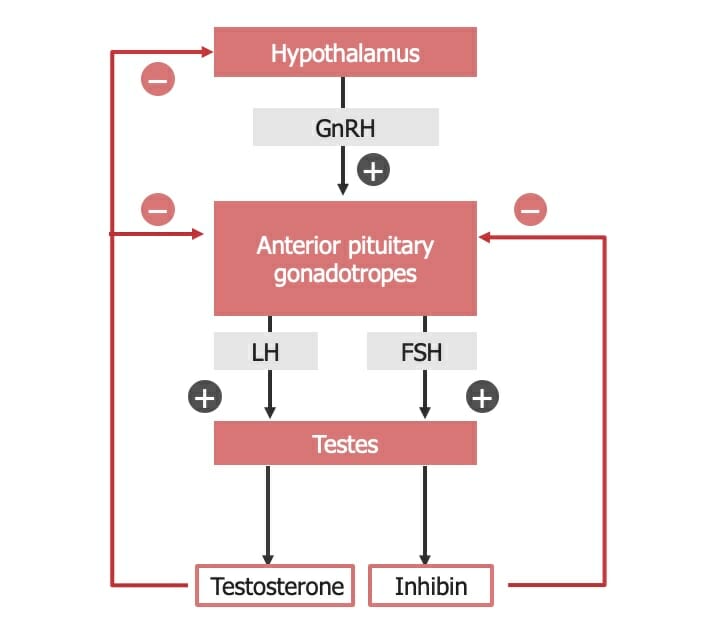
Hypothalamic-pituitary-testicular axis
FSH: follicle-stimulating hormone
GnRH: gonadotropin-releasing hormone
LH: luteinizing hormone
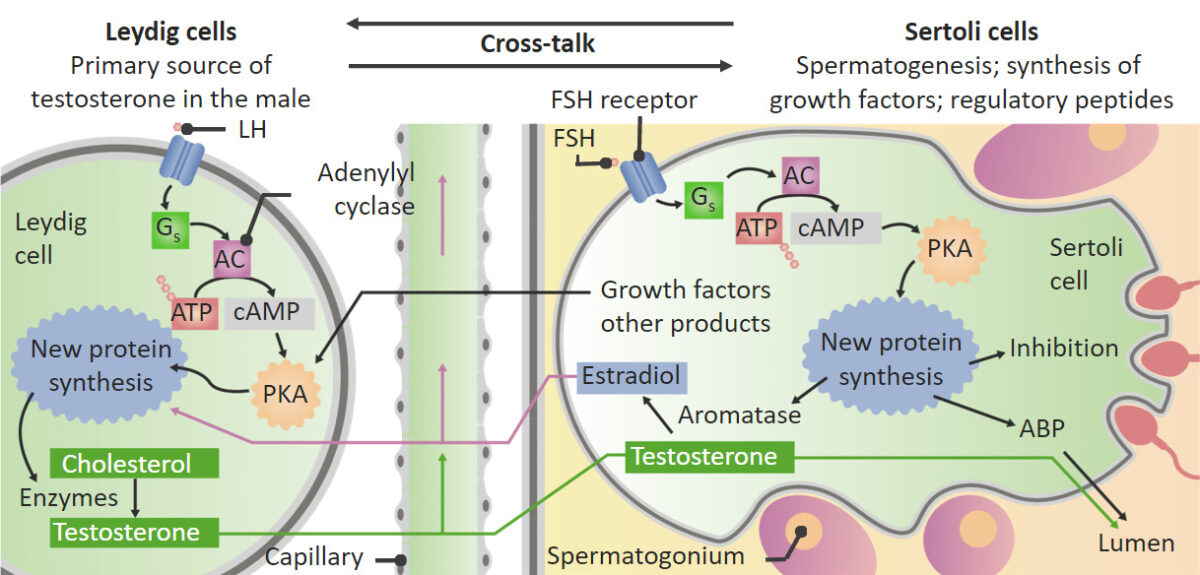
Actions of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) on the testes:
: Within the Leydig cells, LH stimulates the conversion of cholesterol into testosterone. Testosterone then moves into the Sertoli cells and helps to stimulate spermatogenesis. In addition to stimulating spermatogenesis, FSH stimulates the Sertoli cells to produce aromatase, growth factors, and other regulatory peptides. The aromatase converts some testosterone into estrogen. Some estrogen and growth factors produced in the Sertoli cells then move back to the Leydig cells and stimulate the Leydig cells to increase production of testosterone.
ABP: androgen-binding protein
PKA: protein kinase A
Testosterone Testosterone A potent androgenic steroid and major product secreted by the leydig cells of the testis. Its production is stimulated by luteinizing hormone from the pituitary gland. In turn, testosterone exerts feedback control of the pituitary LH and FSH secretion. Depending on the tissues, testosterone can be further converted to dihydrotestosterone or estradiol. Androgens and Antiandrogens, and other androgens Androgens Androgens are naturally occurring steroid hormones responsible for development and maintenance of the male sex characteristics, including penile, scrotal, and clitoral growth, development of sexual hair, deepening of the voice, and musculoskeletal growth. Androgens and Antiandrogens, are responsible for the development of both primary and secondary male sex Sex The totality of characteristics of reproductive structure, functions, phenotype, and genotype, differentiating the male from the female organism. Gender Dysphoria characteristics.
Testosterone Testosterone A potent androgenic steroid and major product secreted by the leydig cells of the testis. Its production is stimulated by luteinizing hormone from the pituitary gland. In turn, testosterone exerts feedback control of the pituitary LH and FSH secretion. Depending on the tissues, testosterone can be further converted to dihydrotestosterone or estradiol. Androgens and Antiandrogens secretion Secretion Coagulation Studies varies throughout the male life span:

Average testosterone concentrations over a life span
Image by Lecturio.
Structure of an antral follicle
Image by Lecturio.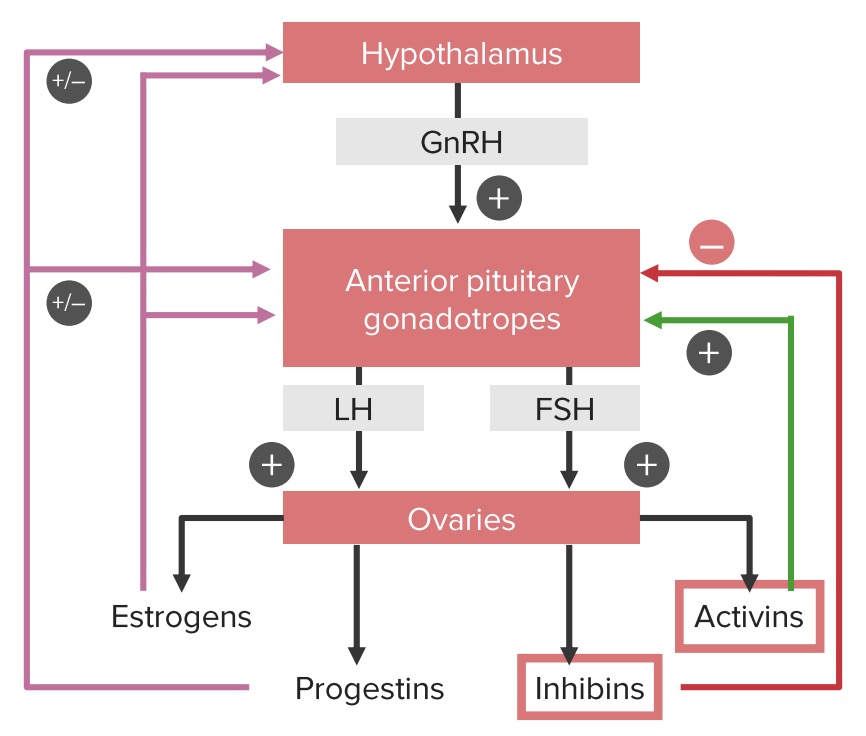
Positive and negative feedback loops of the hypothalamic-pituitary-ovarian axis:
Note that estrogens and progestins can have both a positive and a negative influence on the hypothalamus and pituitary gland, depending on the phase of the cycle. Estrogens provide negative feedback until the middle of the cycle. At this point, estrogen begins stimulating the gonadotropic cells in the pituitary, leading to the luteinizing hormone (LH) surge, which triggers ovulation.
FSH: follicle-stimulating hormone
GnRH: gonadotropin-releasing hormone
GnRH is released in a pulsatile fashion, following multiple biologic rhythms:
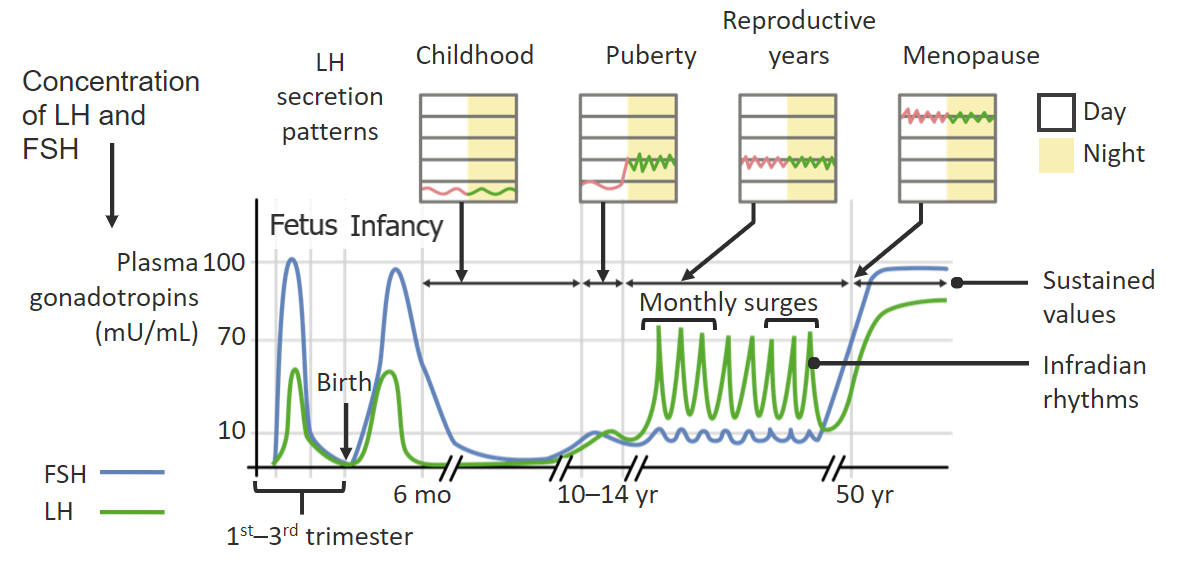
Changes in pulsatile secretion of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) throughout the day and across the span of a female’s life:
Pulsatile release of FSH and LH from the pituitary gland occurs in response to the pulsatile release of gonadotropin-releasing hormone (GnRH) from the hypothalamus.
Review of the sex Sex The totality of characteristics of reproductive structure, functions, phenotype, and genotype, differentiating the male from the female organism. Gender Dysphoria hormone metabolic pathway: cholesterol Cholesterol The principal sterol of all higher animals, distributed in body tissues, especially the brain and spinal cord, and in animal fats and oils. Cholesterol Metabolism → pregnenolone → progesterone → 17α-OHP → androstenedione Androstenedione A delta-4 C19 steroid that is produced not only in the testis, but also in the ovary and the adrenal cortex. Depending on the tissue type, androstenedione can serve as a precursor to testosterone as well as estrone and estradiol. Androgens and Antiandrogens → testosterone Testosterone A potent androgenic steroid and major product secreted by the leydig cells of the testis. Its production is stimulated by luteinizing hormone from the pituitary gland. In turn, testosterone exerts feedback control of the pituitary LH and FSH secretion. Depending on the tissues, testosterone can be further converted to dihydrotestosterone or estradiol. Androgens and Antiandrogens or E1 E1 An aromatized C18 steroid with a 3-hydroxyl group and a 17-ketone, a major mammalian estrogen. It is converted from androstenedione directly, or from testosterone via estradiol. In humans, it is produced primarily by the cyclic ovaries, placenta, and the adipose tissue of men and postmenopausal women. Noncontraceptive Estrogen and Progestins → E2
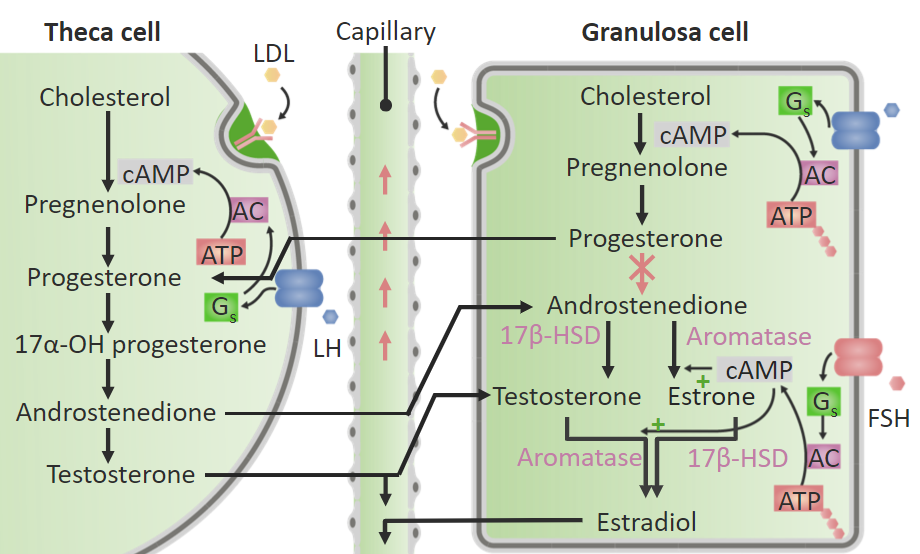
Synthesis of estrogen and testosterone in the ovary:
Different enzymes are present in theca and granulosa cells. Theca cells contain the enzymes necessary to convert cholesterol into testosterone; however, theca cells do not contain aromatase, which is required to convert androgens into estrogens. Therefore, the androgens (androstenedione and testosterone) move from theca cells to aromatase-containing granulosa cells. Within the granulosa cells, aromatase converts testosterone into estradiol (E2) and androstenedione into estrone (E1). The granulosa cells lack the enzyme required to convert progesterone into androstenedione, so the granulosa cells produce both progesterone and estrogens.
AC: adenylyl cyclase
17β-HSD: 17β-hydroxysteroid dehydrogenase
Estrogens play a major role in the sexual development of females and the menstrual cycle Menstrual cycle The menstrual cycle is the cyclic pattern of hormonal and tissular activity that prepares a suitable uterine environment for the fertilization and implantation of an ovum. The menstrual cycle involves both an endometrial and ovarian cycle that are dependent on one another for proper functioning. There are 2 phases of the ovarian cycle and 3 phases of the endometrial cycle. Menstrual Cycle; however, there are numerous nonreproductive functions of estrogens as well.
Progesterone is primarily produced by the corpus luteum Corpus Luteum The yellow body derived from the ruptured ovarian follicle after ovulation. The process of corpus luteum formation, luteinization, is regulated by luteinizing hormone. Ovaries: Anatomy after ovulation Ovulation The discharge of an ovum from a rupturing follicle in the ovary. Menstrual Cycle.
| Male | Female | |
|---|---|---|
| Gonad | Testis | Ovary |
| Germ cells Germ Cells The reproductive cells in multicellular organisms at various stages during gametogenesis. Gametogenesis | Spermatozoa | Ova |
| Enclosure | Seminiferous tubule | Follicle |
| Adjacent cells | Sertoli cells Sertoli Cells Supporting cells projecting inward from the basement membrane of seminiferous tubules. They surround and nourish the developing male germ cells and secrete androgen-binding protein and hormones such as anti-mullerian hormone. The tight junctions of sertoli cells with the spermatogonia and spermatocytes provide a blood-testis barrier. Testicles: Anatomy | Granulosa cells |
| Adjacent cell products |
|
|
| Interstitium | Leydig cells Leydig Cells Steroid-producing cells in the interstitial tissue of the testis. They are under the regulation of pituitary hormones; luteinizing hormone; or interstitial cell-stimulating hormone. Testosterone is the major androgen (androgens) produced. Testicles: Anatomy | Theca cells Theca cells The flattened stroma cells forming a sheath or theca outside the basal lamina lining the mature ovarian follicle. Thecal interstitial or stromal cells are steroidogenic, and produce primarily androgens which serve as precursors of estrogens in the granulosa cells. Puberty |
| Interstitial products | Testosterone Testosterone A potent androgenic steroid and major product secreted by the leydig cells of the testis. Its production is stimulated by luteinizing hormone from the pituitary gland. In turn, testosterone exerts feedback control of the pituitary LH and FSH secretion. Depending on the tissues, testosterone can be further converted to dihydrotestosterone or estradiol. Androgens and Antiandrogens |
|
| Organ system | Estrogens | Androgens Androgens Androgens are naturally occurring steroid hormones responsible for development and maintenance of the male sex characteristics, including penile, scrotal, and clitoral growth, development of sexual hair, deepening of the voice, and musculoskeletal growth. Androgens and Antiandrogens |
|---|---|---|
| Reproductive organs |
|
|
| Dermatologic effects (in all people) | Mild thickening of the skin Skin The skin, also referred to as the integumentary system, is the largest organ of the body. The skin is primarily composed of the epidermis (outer layer) and dermis (deep layer). The epidermis is primarily composed of keratinocytes that undergo rapid turnover, while the dermis contains dense layers of connective tissue. Skin: Structure and Functions |
|
| Musculoskeletal effects |
|
|
| Other effects |
|
|