Statins are competitive inhibitors of HMG-CoA reductase HMG-CoA reductase Enzymes that catalyze the reversible reduction of alpha-carboxyl group of 3-hydroxy-3-methylglutaryl-coenzyme A to yield mevalonic acid. Cholesterol Metabolism in the liver Liver The liver is the largest gland in the human body. The liver is found in the superior right quadrant of the abdomen and weighs approximately 1.5 kilograms. Its main functions are detoxification, metabolism, nutrient storage (e.g., iron and vitamins), synthesis of coagulation factors, formation of bile, filtration, and storage of blood. Liver: Anatomy. HMG-CoA reductase HMG-CoA reductase Enzymes that catalyze the reversible reduction of alpha-carboxyl group of 3-hydroxy-3-methylglutaryl-coenzyme A to yield mevalonic acid. Cholesterol Metabolism is the rate-limiting step in cholesterol Cholesterol The principal sterol of all higher animals, distributed in body tissues, especially the brain and spinal cord, and in animal fats and oils. Cholesterol Metabolism synthesis Synthesis Polymerase Chain Reaction (PCR). Inhibition results in lowered intrahepatocytic cholesterol Cholesterol The principal sterol of all higher animals, distributed in body tissues, especially the brain and spinal cord, and in animal fats and oils. Cholesterol Metabolism formation, resulting in up-regulation Up-Regulation A positive regulatory effect on physiological processes at the molecular, cellular, or systemic level. At the molecular level, the major regulatory sites include membrane receptors, genes (gene expression regulation), mRNAs, and proteins. Pharmacokinetics and Pharmacodynamics of LDL receptors Receptors Receptors are proteins located either on the surface of or within a cell that can bind to signaling molecules known as ligands (e.g., hormones) and cause some type of response within the cell. Receptors and, ultimately, lowering levels of serum LDL and triglycerides Triglycerides Fatty Acids and Lipids. Statins can lower LDL 20%–60% (depending on their intensity) and have benefits that are cholesterol-independent (e.g., reduced vascular inflammation Inflammation Inflammation is a complex set of responses to infection and injury involving leukocytes as the principal cellular mediators in the body's defense against pathogenic organisms. Inflammation is also seen as a response to tissue injury in the process of wound healing. The 5 cardinal signs of inflammation are pain, heat, redness, swelling, and loss of function. Inflammation and atherosclerotic plaque Plaque Primary Skin Lesions stabilization). Indications for prescribing statins include prevention of primary or secondary cardiovascular disease in patients Patients Individuals participating in the health care system for the purpose of receiving therapeutic, diagnostic, or preventive procedures. Clinician–Patient Relationship with dyslipidemia. The main adverse effects are transaminitis Transaminitis Tick-borne Encephalitis Virus and muscle toxicity Toxicity Dosage Calculation.
Last updated: Mar 27, 2025
Statins are competitive inhibitors of the enzyme HMG-CoA reductase HMG-CoA reductase Enzymes that catalyze the reversible reduction of alpha-carboxyl group of 3-hydroxy-3-methylglutaryl-coenzyme A to yield mevalonic acid. Cholesterol Metabolism, which is the rate-limiting step in cholesterol Cholesterol The principal sterol of all higher animals, distributed in body tissues, especially the brain and spinal cord, and in animal fats and oils. Cholesterol Metabolism biosynthesis Biosynthesis The biosynthesis of peptides and proteins on ribosomes, directed by messenger RNA, via transfer RNA that is charged with standard proteinogenic amino acids. Virology in the liver Liver The liver is the largest gland in the human body. The liver is found in the superior right quadrant of the abdomen and weighs approximately 1.5 kilograms. Its main functions are detoxification, metabolism, nutrient storage (e.g., iron and vitamins), synthesis of coagulation factors, formation of bile, filtration, and storage of blood. Liver: Anatomy.
Statins are structural analogs of HMG-CoA.
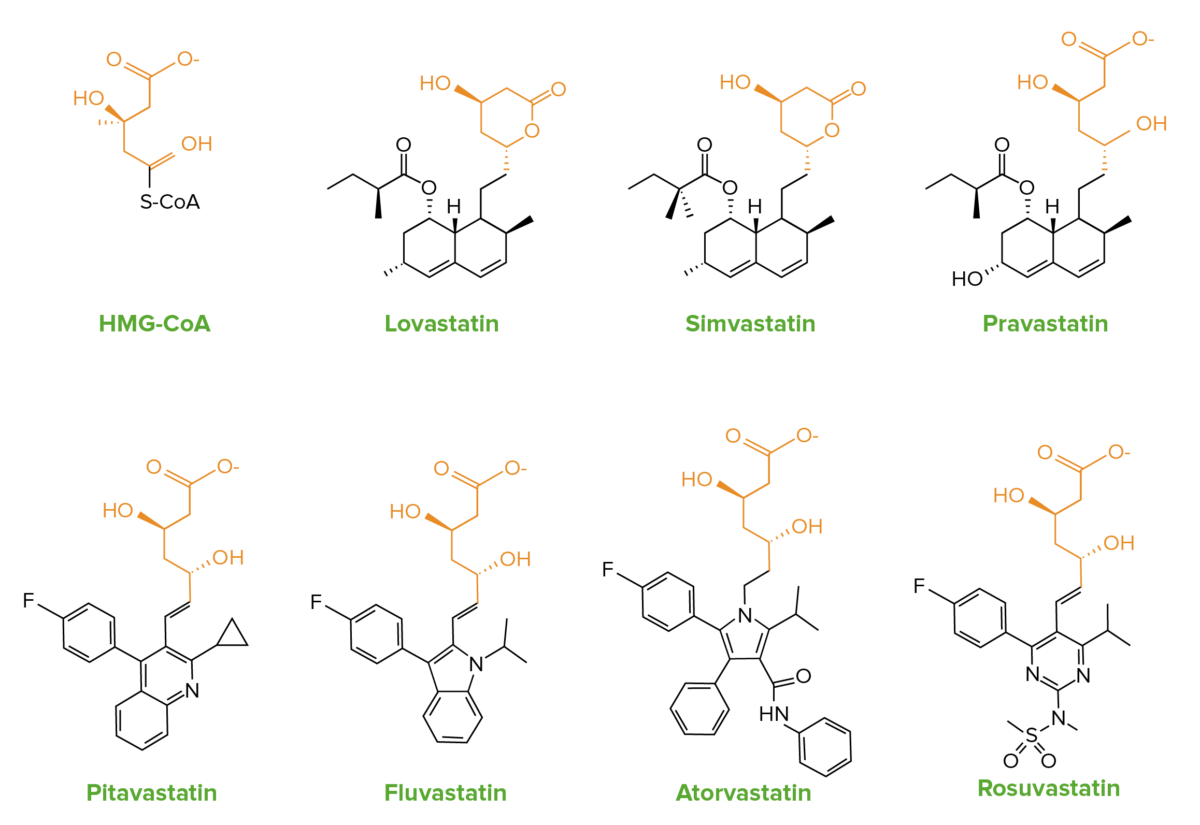
The similarities in chemical structure of statins to HMG-CoA:
Lovastatin and simvastatin contain an inactive lactone ring, which is hydrolyzed in the body to the active form.
Normal physiology:
Statins:
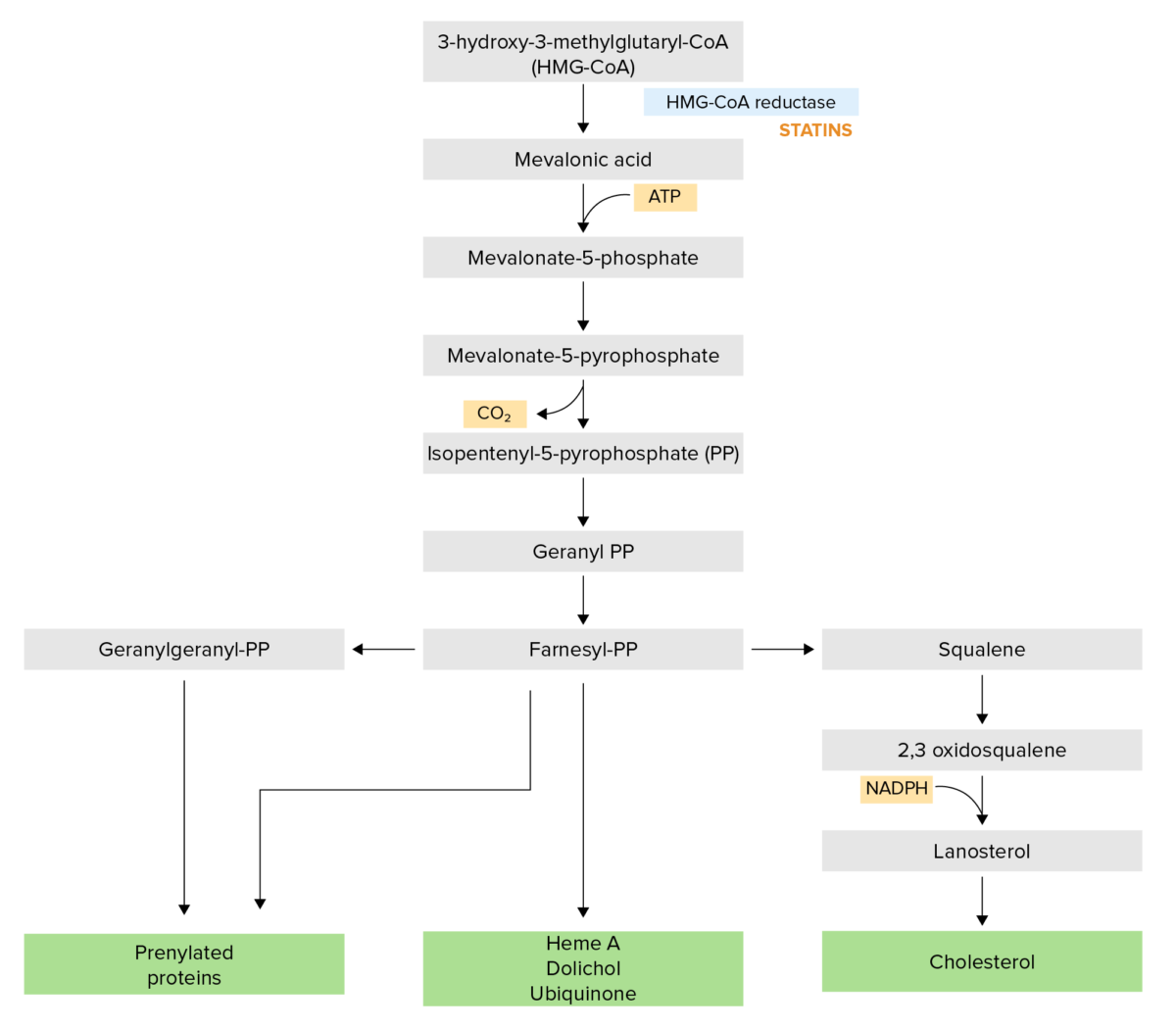
The cholesterol synthesis pathway:
Statins competitively inhibit HMG-CoA reductase, which is a rate-limiting step in the pathway.
NADPH: nicotinamide adenine dinucleotide phosphate
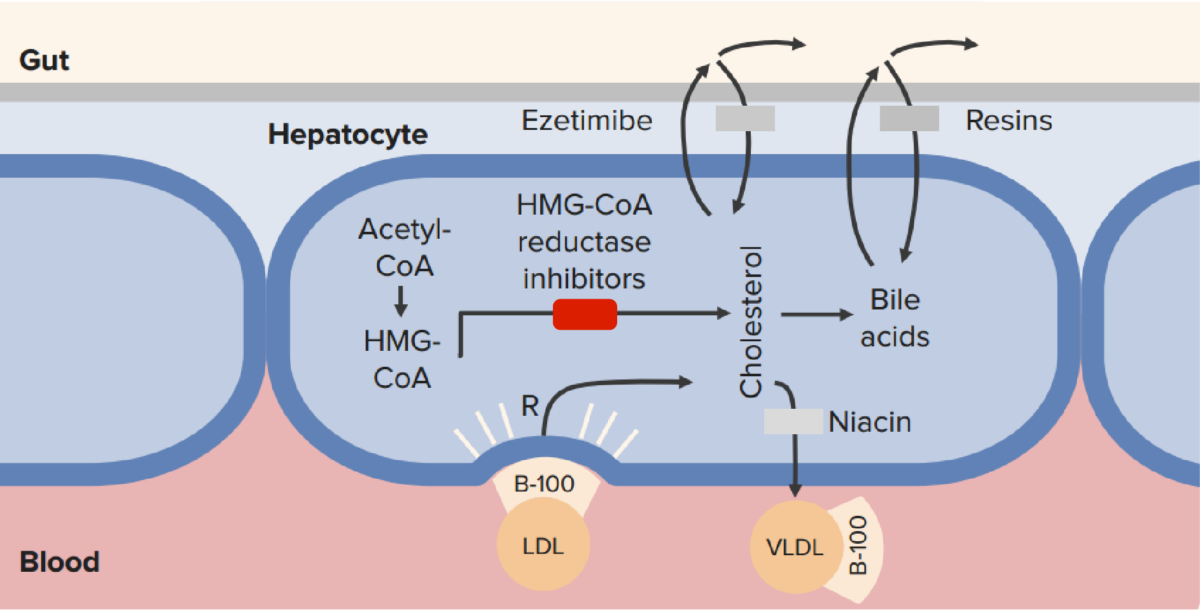
The mechanism of action of statins (HMG-CoA reductase inhibitors) in comparison to other lipid-lowering therapies:
Statins block intrahepatocyte synthesis of cholesterol, resulting in up-regulation of LDL receptors and a reduction in serum LDL.
CoA: coenzyme A
Statins are classified on the basis of their percent reduction of LDL and dosing:
Statins are indicated to treat dyslipidemias Dyslipidemias Abnormalities in the serum levels of lipids, including overproduction or deficiency. Abnormal serum lipid profiles may include high total cholesterol, high triglycerides, low high density lipoprotein cholesterol, and elevated low density lipoprotein cholesterol. Lipid Disorders with the intent of reducing major adverse cardiovascular events:
The following table compares and contrasts members of the statin drug class. The drugs are listed in descending order of potency.
| Statin | Solubility | Metabolism | Plasma Plasma The residual portion of blood that is left after removal of blood cells by centrifugation without prior blood coagulation. Transfusion Products half-life Half-Life The time it takes for a substance (drug, radioactive nuclide, or other) to lose half of its pharmacologic, physiologic, or radiologic activity. Pharmacokinetics and Pharmacodynamics | Optimal dosing time |
|---|---|---|---|---|
| Pitavastatin | Lipophilic | CYP2C9 CYP2C9 A cytochrome p-450 subtype that has specificity for acidic xenobiotics. It oxidizes a broad range of important clinical drugs that fall under the categories of nonsteroidal anti-inflammatory agents; hypoglycemic agents; anticoagulants; and diuretics. Anticoagulants | 12 hours | Anytime |
| Rosuvastatin | Hydrophilic Hydrophilic Aminoglycosides | CYP2C9 CYP2C9 A cytochrome p-450 subtype that has specificity for acidic xenobiotics. It oxidizes a broad range of important clinical drugs that fall under the categories of nonsteroidal anti-inflammatory agents; hypoglycemic agents; anticoagulants; and diuretics. Anticoagulants | 19 hours | Anytime |
| Atorvastatin | Lipophilic | CYP3A4 CYP3A4 Class 3 Antiarrhythmic Drugs (Potassium Channel Blockers) | 14 hours | Anytime |
| Lovastatin | Lipophilic | CYP3A4 CYP3A4 Class 3 Antiarrhythmic Drugs (Potassium Channel Blockers) | 2‒3 hours | Evening |
| Simvastatin | Lipophilic | CYP3A4 CYP3A4 Class 3 Antiarrhythmic Drugs (Potassium Channel Blockers) | 2‒3 hours | Evening |
| Pravastatin | Hydrophilic Hydrophilic Aminoglycosides | Sulfation | 1‒3 hours | Anytime |
| Fluvastatin | Lipophilic | CYP2C9 CYP2C9 A cytochrome p-450 subtype that has specificity for acidic xenobiotics. It oxidizes a broad range of important clinical drugs that fall under the categories of nonsteroidal anti-inflammatory agents; hypoglycemic agents; anticoagulants; and diuretics. Anticoagulants | 1‒3 hours | Evening |