Lipids are a diverse group of hydrophobic organic molecules, which include fats, oils, sterols, and waxes. Fatty acids (FAs) are integral building blocks of lipids, and can be classified as unsaturated or saturated based on the presence/absence of carbon-carbon double bonds within their nonpolar chains. Eicosanoids are a family of cell-signaling molecules with important physiologic properties derived from the fatty acid, arachidonic acid. In addition, combining fatty acids with different bases, including glycerol, phosphate, and sphingosine, results in different lipids with varied functions within the human body. Glycerolipids (triacylglycerols) are important for energy storage and thermal insulation. Glycerophospholipids and sphingolipids are essential constituents of cellular plasma membranes. Another group of lipids is based off of isoprenoids, which are the building blocks of sterols (such as cholesterol). Altered levels of lipids (both an overabundance or deficiency) can result in many potential disease processes.
Last updated: Apr 24, 2025
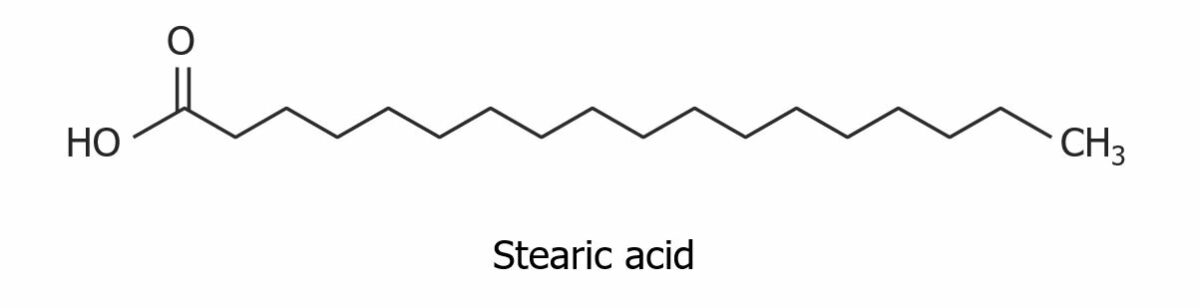
Structure of stearic acid, a saturated fatty acid
Image by Lecturio.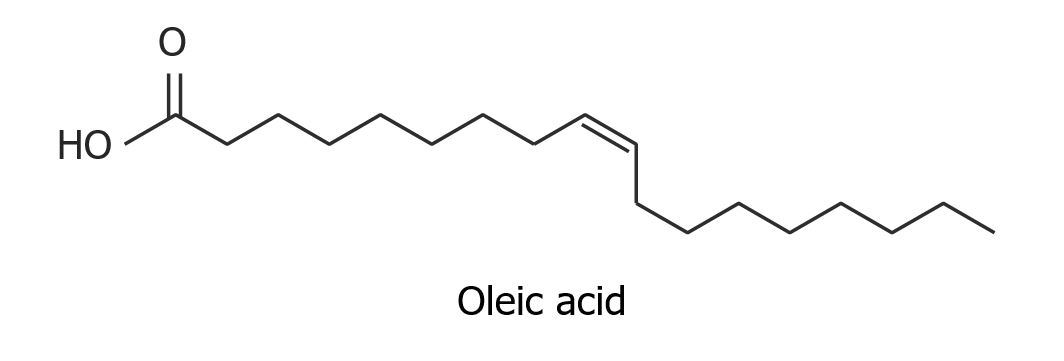
Structure of oleic acid, an unsaturated fatty acid:
Notice the cis configuration of the double bond.
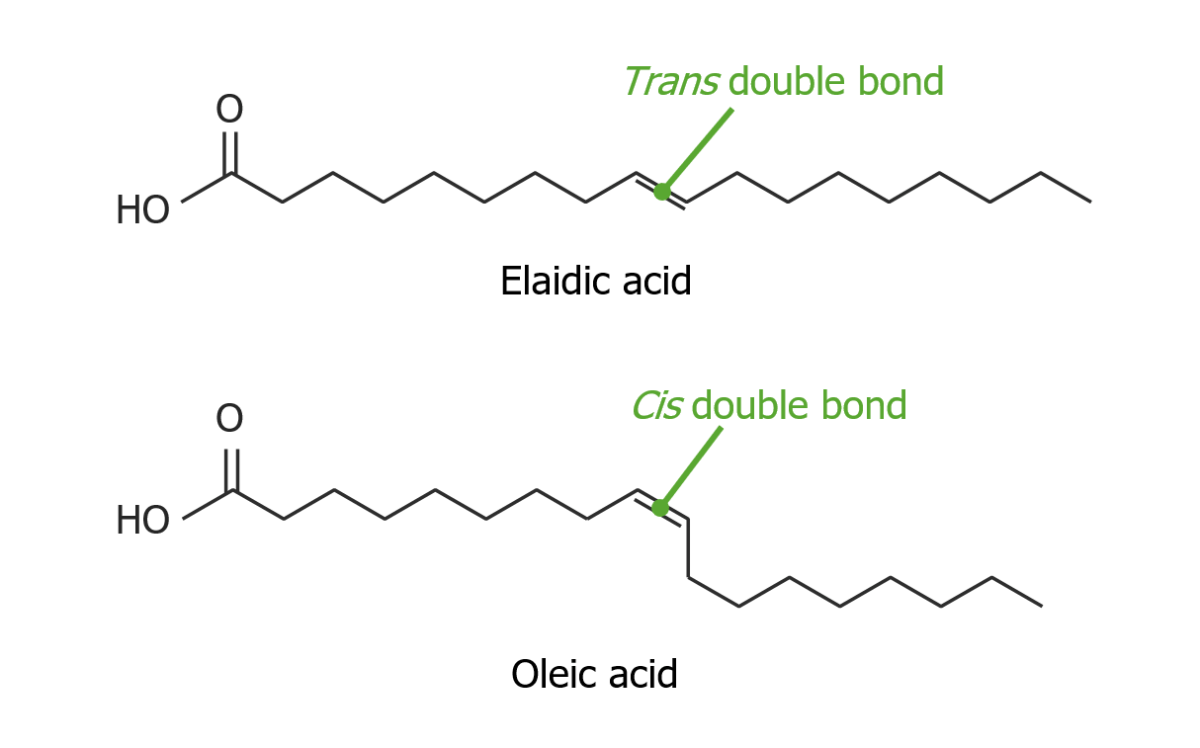
Comparison between the double bond of elaidic acid (trans configuration) and the double bond of oleic acid (cis configuration)
Image by Lecturio.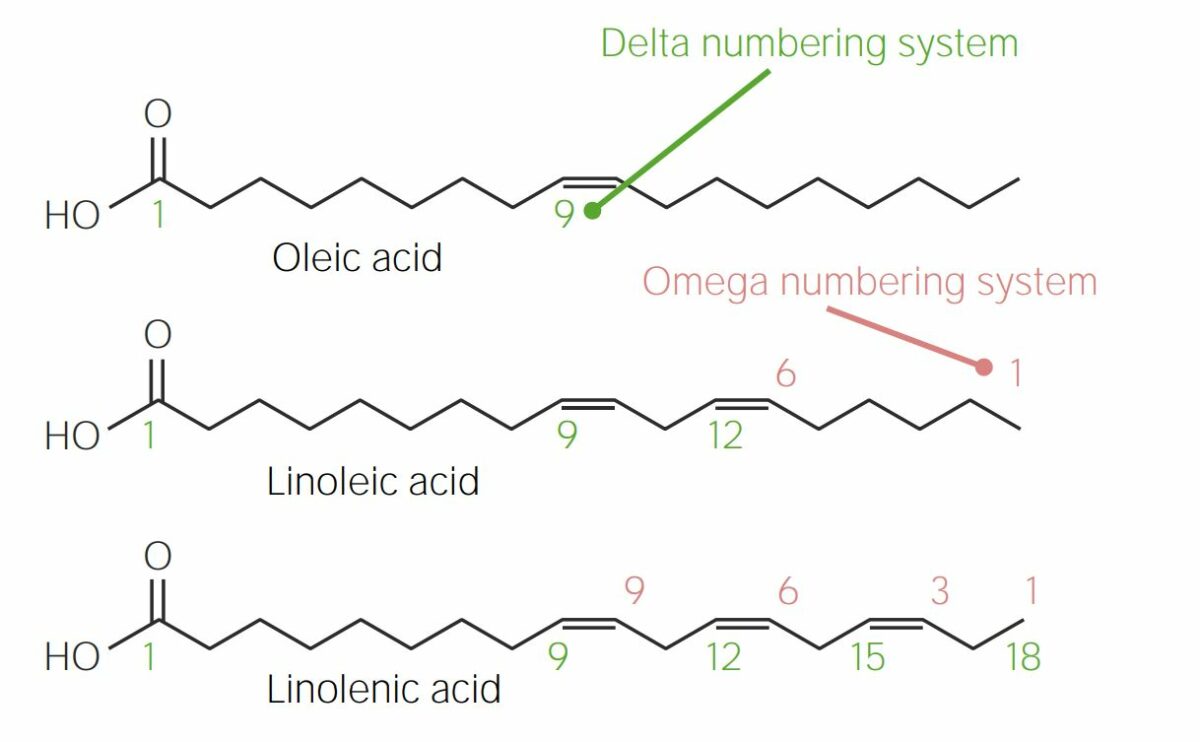
Comparison between the delta numbering system (left to right) and the omega numbering system (right to left):
Note that linoleic acid is an omega-6 fatty acid, while linolenic acid is classified as an omega-3 fatty acid.
| Name | Number of carbon atoms | Sources |
|---|---|---|
| Lauric acid | 12 | Palm kernel oil, coconut oil, laurels, and butter |
| Myristic acid | 14 | Nutmeg, palm kernel, coconut oil, and butter |
| Palmitic acid | 16 | Found in many animal and plant fats Fats The glyceryl esters of a fatty acid, or of a mixture of fatty acids. They are generally odorless, colorless, and tasteless if pure, but they may be flavored according to origin. Fats are insoluble in water, soluble in most organic solvents. They occur in animal and vegetable tissue and are generally obtained by boiling or by extraction under pressure. They are important in the diet (dietary fats) as a source of energy. Energy Homeostasis |
| Stearic acid | 18 | Animal fats Fats The glyceryl esters of a fatty acid, or of a mixture of fatty acids. They are generally odorless, colorless, and tasteless if pure, but they may be flavored according to origin. Fats are insoluble in water, soluble in most organic solvents. They occur in animal and vegetable tissue and are generally obtained by boiling or by extraction under pressure. They are important in the diet (dietary fats) as a source of energy. Energy Homeostasis, cocoa, and shea butters |
| Common name | Classification | # of carbons: # of double bonds (D); orientation- locations of D1, D2… | Family | Notes |
|---|---|---|---|---|
| Oleic acid | Monoenoic acids Acids Chemical compounds which yield hydrogen ions or protons when dissolved in water, whose hydrogen can be replaced by metals or basic radicals, or which react with bases to form salts and water (neutralization). An extension of the term includes substances dissolved in media other than water. Acid-Base Balance | 18:1; cis CIS Multiple Sclerosis-9 | ω9 |
|
| Linoleic acid | Dienoic acids Acids Chemical compounds which yield hydrogen ions or protons when dissolved in water, whose hydrogen can be replaced by metals or basic radicals, or which react with bases to form salts and water (neutralization). An extension of the term includes substances dissolved in media other than water. Acid-Base Balance | 18:2; cis CIS Multiple Sclerosis-9,12 | ω6 |
|
| α-linolenic acid (ALA) | Trienoic acids Acids Chemical compounds which yield hydrogen ions or protons when dissolved in water, whose hydrogen can be replaced by metals or basic radicals, or which react with bases to form salts and water (neutralization). An extension of the term includes substances dissolved in media other than water. Acid-Base Balance | 18:3; cis CIS Multiple Sclerosis-9,12,15 | ω3 |
|
| Arachidonic acid Arachidonic Acid An unsaturated, essential fatty acid. It is found in animal and human fat as well as in the liver, brain, and glandular organs, and is a constituent of animal phosphatides. It is formed by the synthesis from dietary linoleic acid and is a precursor in the biosynthesis of prostaglandins, thromboxanes, and leukotrienes. Nonsteroidal Antiinflammatory Drugs (NSAIDs) | Tetraenoic acids Acids Chemical compounds which yield hydrogen ions or protons when dissolved in water, whose hydrogen can be replaced by metals or basic radicals, or which react with bases to form salts and water (neutralization). An extension of the term includes substances dissolved in media other than water. Acid-Base Balance | 20:4; cis CIS Multiple Sclerosis-5,8,11,14 | ω6 |
|
| Eicosapentaenoic acid (EPA) | Pentanoic acids Acids Chemical compounds which yield hydrogen ions or protons when dissolved in water, whose hydrogen can be replaced by metals or basic radicals, or which react with bases to form salts and water (neutralization). An extension of the term includes substances dissolved in media other than water. Acid-Base Balance | 20:5; cis CIS Multiple Sclerosis-5,8,11,14,17 | ω3 |
|
| Docosahexaenoic acid (DHA) | Hexanoic acids Acids Chemical compounds which yield hydrogen ions or protons when dissolved in water, whose hydrogen can be replaced by metals or basic radicals, or which react with bases to form salts and water (neutralization). An extension of the term includes substances dissolved in media other than water. Acid-Base Balance | 22:6; cis CIS Multiple Sclerosis-4,7,10,13,16,19 | ω3 |
|
Glycerolipids and glycerophospholipids are glycerol-based lipids ubiquitous in human cells. They function as structural components of plasma Plasma The residual portion of blood that is left after removal of blood cells by centrifugation without prior blood coagulation. Transfusion Products membranes and as storage for energy.
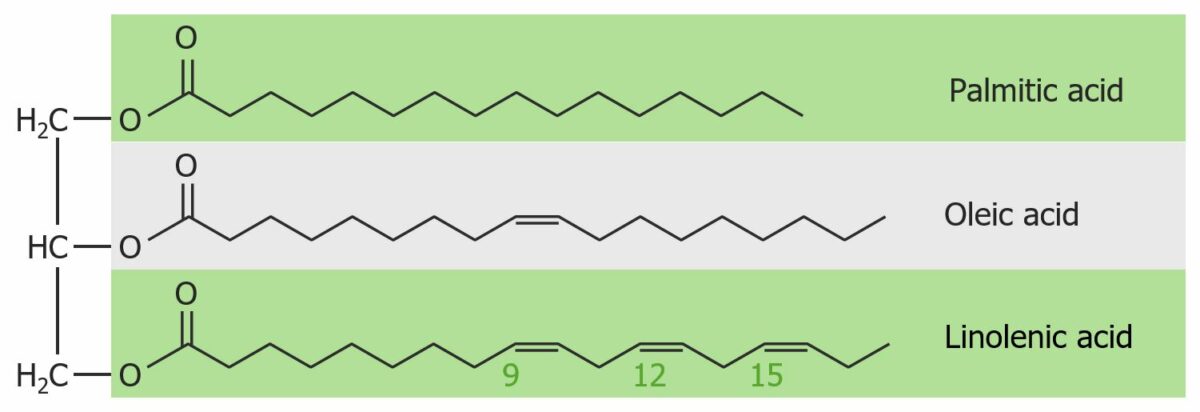
Structure of a triacylglycerol molecule:
See its glycerol backbone and its constituent fatty acids. Linolenic acid is more susceptible to oxidation than the other 2 fatty acids due to its extra double bonds. This decomposition causes the fat to become rancid, i.e., to have an unpleasant taste or smell.
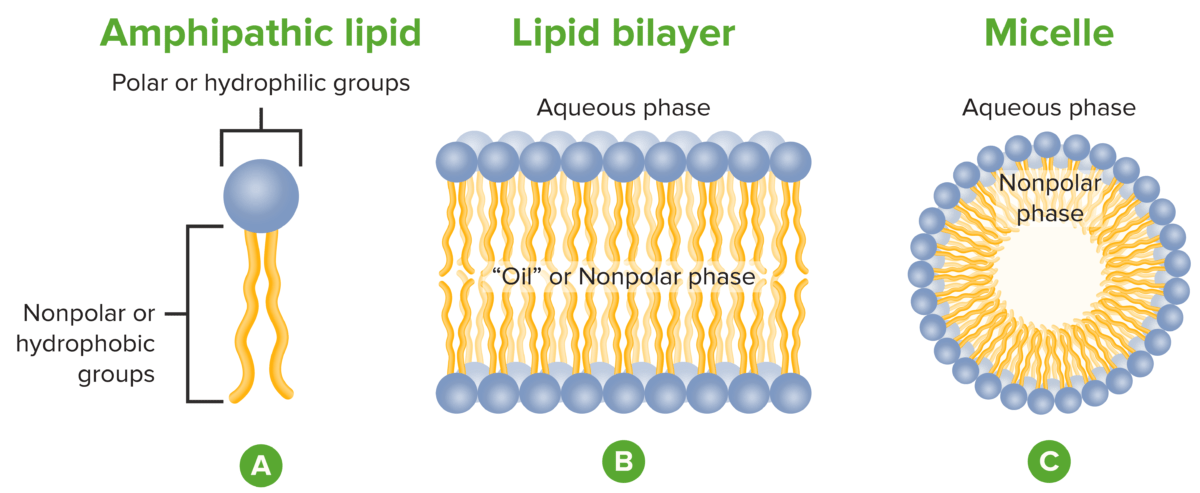
Lipid arrangements:
Glycerophospholipids (i.e., phospholipids) contain a polar head and nonpolar tail (A).
A lipid bilayer is composed of glycerophospholipid molecules with their heads facing outward, and tails inward (B).
This arrangement is usually preferred by glycerophospholipids because their hydrophobic tails are often too bulky for the spherical shape of a micelle (C).
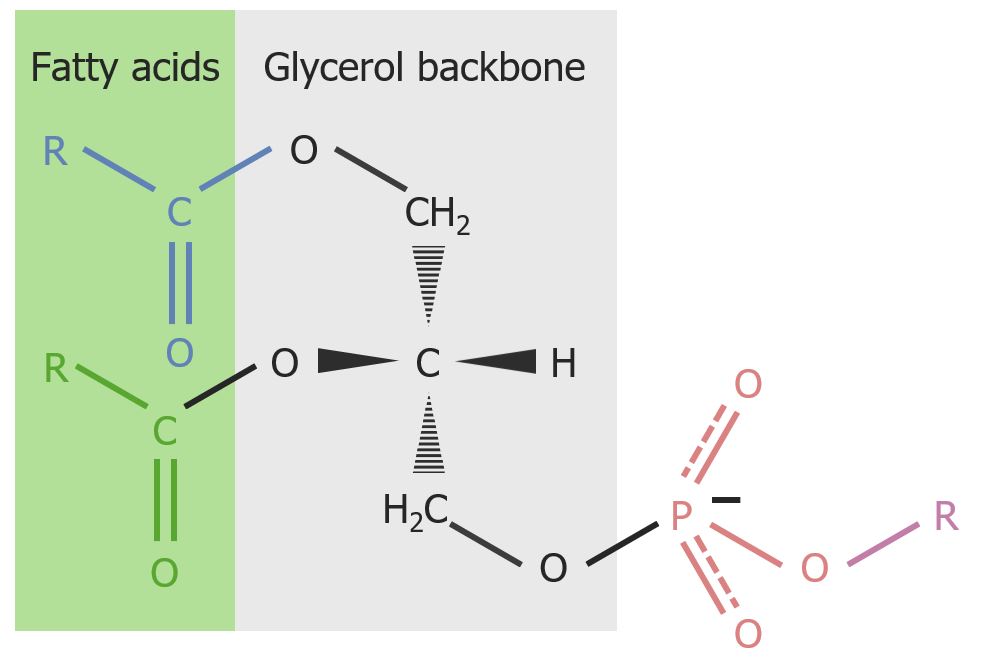
The phosphatidyl backbone of glycerophospholipids:
Polar compounds bond to the phosphate group and are represented by “R”.
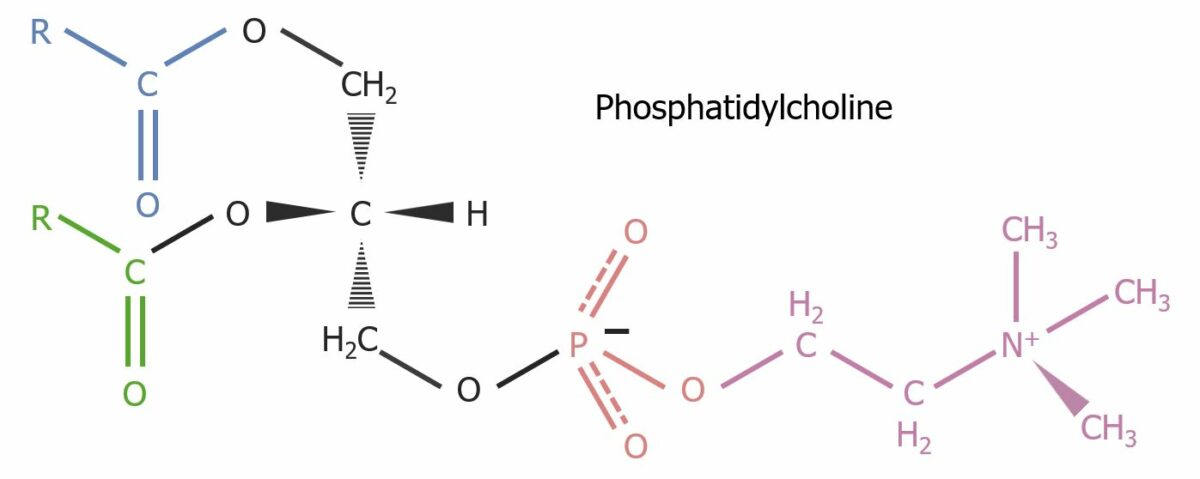
The structure of phosphatidylcholine, a glycerophospholipid constituent of the cell membrane
Image by Lecturio.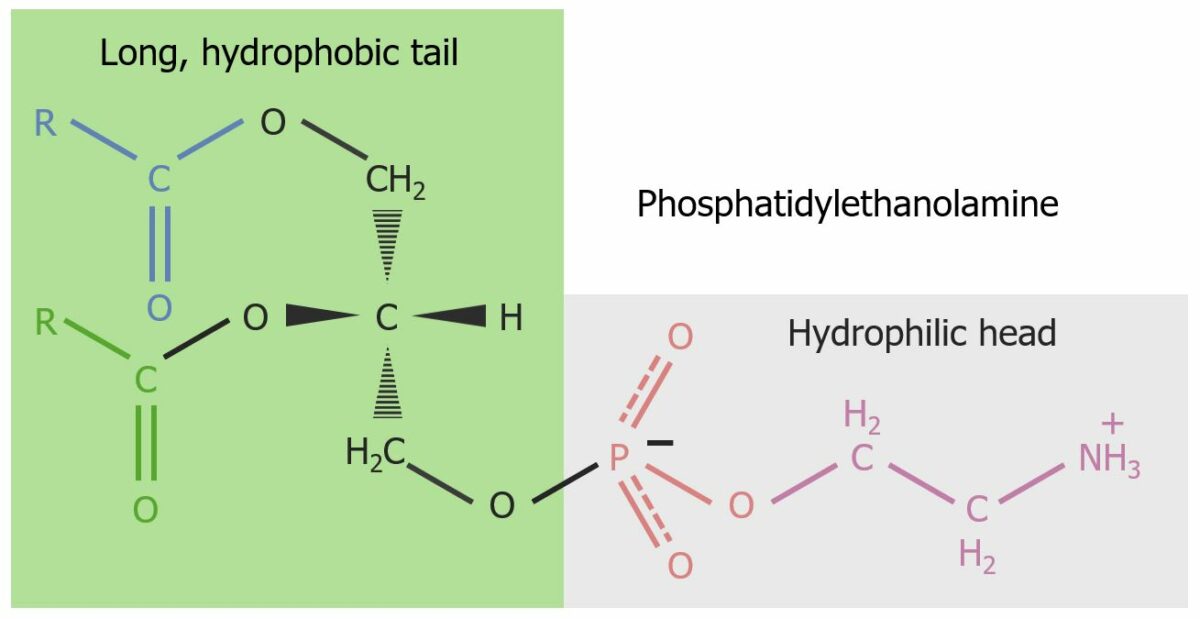
Phosphatidylethanolamine (cephalin): a glycerophospholipid (ethanolamine is linked to the phosphate group) constituent of the cell membrane:
The hydrophilic head and hydrophobic body are highlighted and give rise to the amphiphilic property of the glycerophospholipid. Organized into micelles, structural components of the lipid bilayer are formed.
Sphingolipids are a heterogeneous family of lipids sharing the characteristic sphingoid base backbone:
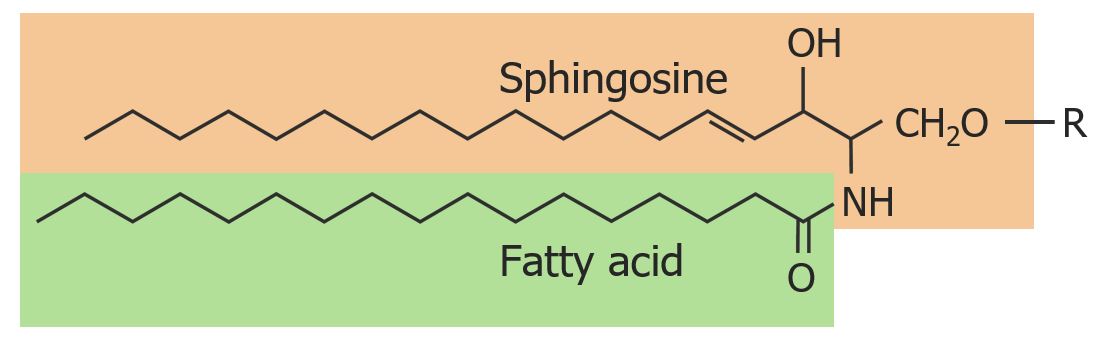
The basic structure of a sphingolipid:
Ceramides consist of a sphingosine backbone bound to a fatty acid and an R group. The “R” represents the compound that will differentiate the different types of sphingolipids. If the R group is simply a hydrogen atom, then the molecule is known as ceramide.
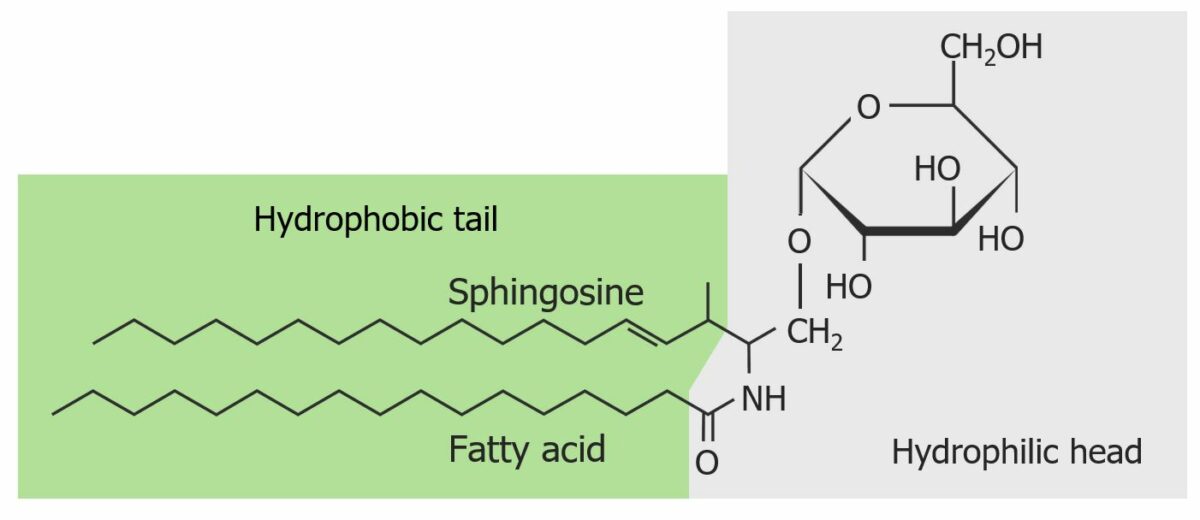
The structure of a cerebroside:
A ceramide (sphingosine + fatty acid) is bound to a monosaccharide (a single simple sugar). In this case, glucose is bound to ceramide, forming glucocerebroside. The hydrophilic (monosaccharide) and hydrophobic (ceramide) ends are highlighted.
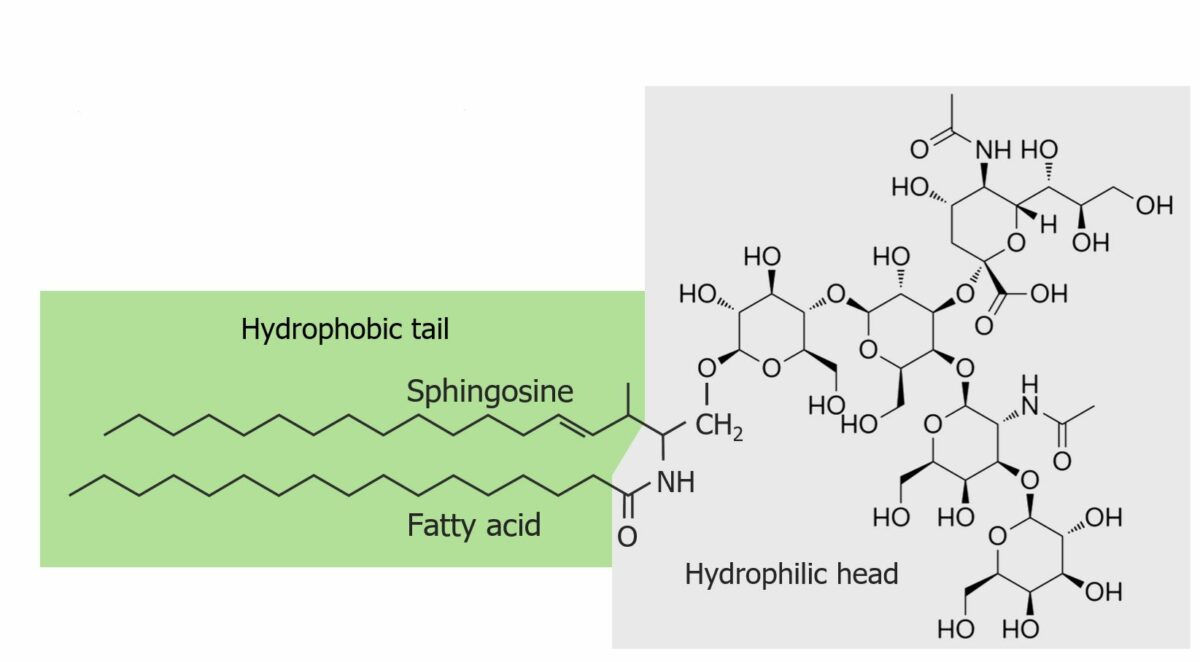
The structure of a ganglioside:
A ceramide (sphingosine + fatty acid) is bound to an oligosaccharide (a carbohydrate containing 3‒10 monosaccharides). The hydrophilic (complex carbohydrate) and hydrophobic (ceramide) ends are highlighted.
Sterols and isoprenoids, which form sterols, are an important group of lipids used as part of the cellular plasma membrane Plasma membrane A cell membrane (also known as the plasma membrane or plasmalemma) is a biological membrane that separates the cell contents from the outside environment. A cell membrane is composed of a phospholipid bilayer and proteins that function to protect cellular DNA and mediate the exchange of ions and molecules. The Cell: Cell Membrane, steroid hormones Steroid hormones Steroid hormones produced by the gonads. They stimulate reproductive organs, germ cell maturation, and the secondary sex characteristics in the males and the females. The major sex steroid hormones include estradiol; progesterone; and testosterone. Hormones: Overview and Types, and as bile Bile An emulsifying agent produced in the liver and secreted into the duodenum. Its composition includes bile acids and salts; cholesterol; and electrolytes. It aids digestion of fats in the duodenum. Gallbladder and Biliary Tract: Anatomy acids Acids Chemical compounds which yield hydrogen ions or protons when dissolved in water, whose hydrogen can be replaced by metals or basic radicals, or which react with bases to form salts and water (neutralization). An extension of the term includes substances dissolved in media other than water. Acid-Base Balance, which help absorb fats Fats The glyceryl esters of a fatty acid, or of a mixture of fatty acids. They are generally odorless, colorless, and tasteless if pure, but they may be flavored according to origin. Fats are insoluble in water, soluble in most organic solvents. They occur in animal and vegetable tissue and are generally obtained by boiling or by extraction under pressure. They are important in the diet (dietary fats) as a source of energy. Energy Homeostasis.
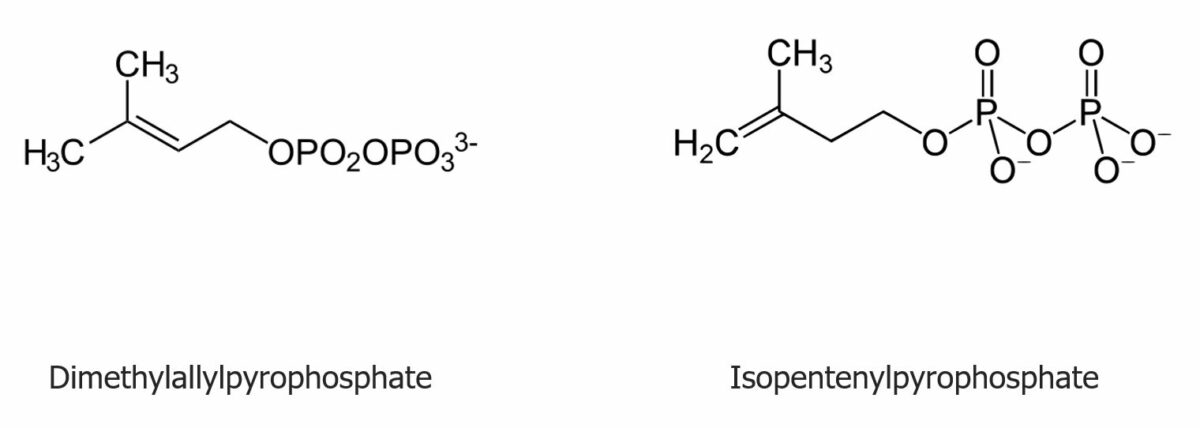
The structure of dimethylallylpyrophosphate and isopentenylpyrophosphate:
The repetitive addition of the molecules ultimately produces the precursors to sterols.
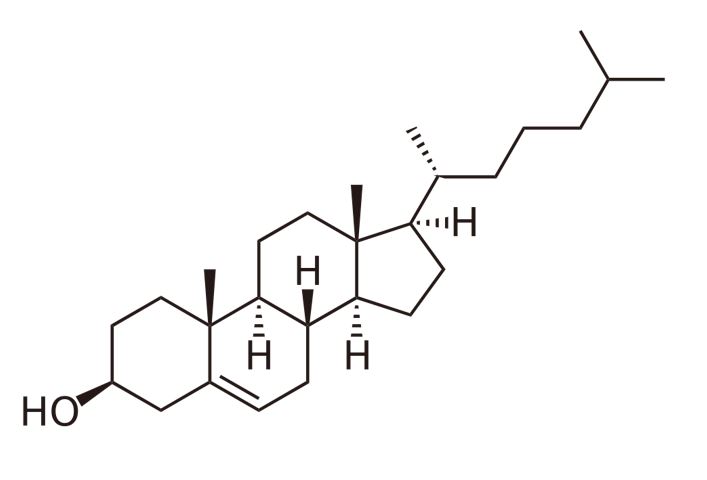
The structure of free cholesterol
Image by Lecturio.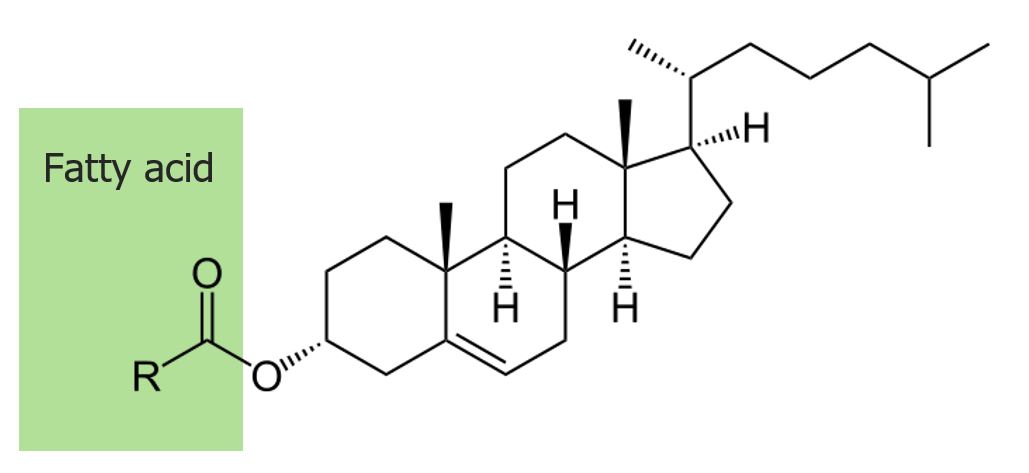
The structure of cholesteryl ester:
By means of an ester bond to a fatty acid, cholesterol is incorporated and stored in the plasma membrane.