Opiates are drugs that are derived from the sap of the opium poppy. Opiates have been used since antiquity for the relief of acute severe pain Pain An unpleasant sensation induced by noxious stimuli which are detected by nerve endings of nociceptive neurons. Pain: Types and Pathways. Opioids are synthetic opiates with properties that are substantially similar to those of opiates. Known for their remarkable efficacy, opioids induce their effects ( analgesia Analgesia Methods of pain relief that may be used with or in place of analgesics. Anesthesiology: History and Basic Concepts, euphoria Euphoria An exaggerated feeling of physical and emotional well-being not consonant with apparent stimuli or events; usually of psychologic origin, but also seen in organic brain disease and toxic states. Hepatic Encephalopathy, and sedation) by interacting with opioid Opioid Compounds with activity like opiate alkaloids, acting at opioid receptors. Properties include induction of analgesia or narcosis. Constipation receptors Receptors Receptors are proteins located either on the surface of or within a cell that can bind to signaling molecules known as ligands (e.g., hormones) and cause some type of response within the cell. Receptors (μ, κ, and δ) in the nervous system Nervous system The nervous system is a small and complex system that consists of an intricate network of neural cells (or neurons) and even more glial cells (for support and insulation). It is divided according to its anatomical components as well as its functional characteristics. The brain and spinal cord are referred to as the central nervous system, and the branches of nerves from these structures are referred to as the peripheral nervous system. Nervous System: Anatomy, Structure, and Classification. Opiates/opioids have adverse effects that include respiratory depression, nausea Nausea An unpleasant sensation in the stomach usually accompanied by the urge to vomit. Common causes are early pregnancy, sea and motion sickness, emotional stress, intense pain, food poisoning, and various enteroviruses. Antiemetics and vomiting Vomiting The forcible expulsion of the contents of the stomach through the mouth. Hypokalemia, decreased GI motility GI Motility The primary functions of the GI tract are digestion and absorption, which require coordinated contractions of the smooth muscles present in the GI tract. Peristaltic waves, segmentation contractions, and the migrating motor complex are all important contraction patterns that help to mix contents, get them in contact with the intestinal walls, and propel material down the tract at appropriate times and in appropriate amounts. Gastrointestinal Motility and constipation Constipation Constipation is common and may be due to a variety of causes. Constipation is generally defined as bowel movement frequency < 3 times per week. Patients who are constipated often strain to pass hard stools. The condition is classified as primary (also known as idiopathic or functional constipation) or secondary, and as acute or chronic. Constipation, tolerance Tolerance Pharmacokinetics and Pharmacodynamics, dependence, and addiction Addiction Substance use disorders are a significant cause of morbidity and mortality, especially among adolescents and young adults. A substance-related and addictive disorder is the continued use of a substance despite harmful consequences; these include significant impairment to one's health or relationships or failure to fulfill major responsibilities at work, school, or home because of substance use. Substance-Related and Addictive Disorders.
Last updated: May 17, 2024
Opioids are a class of natural or synthetic drugs that act on opioid Opioid Compounds with activity like opiate alkaloids, acting at opioid receptors. Properties include induction of analgesia or narcosis. Constipation receptors Receptors Receptors are proteins located either on the surface of or within a cell that can bind to signaling molecules known as ligands (e.g., hormones) and cause some type of response within the cell. Receptors to provide analgesia Analgesia Methods of pain relief that may be used with or in place of analgesics. Anesthesiology: History and Basic Concepts and CNS effects.
The following table summarizes and compares the effects of opioid Opioid Compounds with activity like opiate alkaloids, acting at opioid receptors. Properties include induction of analgesia or narcosis. Constipation receptors Receptors Receptors are proteins located either on the surface of or within a cell that can bind to signaling molecules known as ligands (e.g., hormones) and cause some type of response within the cell. Receptors:
| Mu (μ) receptor Receptor Receptors are proteins located either on the surface of or within a cell that can bind to signaling molecules known as ligands (e.g., hormones) and cause some type of response within the cell. Receptors | Kappa (κ) receptor Receptor Receptors are proteins located either on the surface of or within a cell that can bind to signaling molecules known as ligands (e.g., hormones) and cause some type of response within the cell. Receptors | Delta (δ) receptor Receptor Receptors are proteins located either on the surface of or within a cell that can bind to signaling molecules known as ligands (e.g., hormones) and cause some type of response within the cell. Receptors | |
|---|---|---|---|
| Effect on analgesia Analgesia Methods of pain relief that may be used with or in place of analgesics. Anesthesiology: History and Basic Concepts | Spinal and central | Spinal | Spinal |
| Effect on GI tract | Slowed gastric transit | ||
| Other effects |
|
|
|
| Habituation | None | None | Tolerance Tolerance Pharmacokinetics and Pharmacodynamics |
| Agonists |
|
|
|
CNS:
Peripheral:
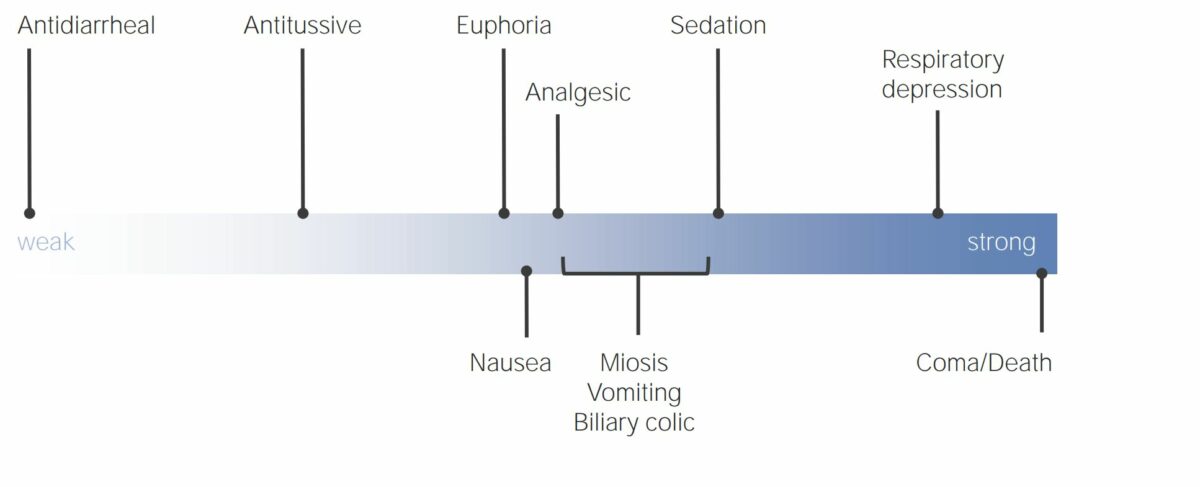
Effects of opioid analgesics in relation to the strength of the drug
Image by Lecturio. License: CC BY-NC-SA 4.0The opioid Opioid Compounds with activity like opiate alkaloids, acting at opioid receptors. Properties include induction of analgesia or narcosis. Constipation analgesics can be classified on the basis of their activity on opioid Opioid Compounds with activity like opiate alkaloids, acting at opioid receptors. Properties include induction of analgesia or narcosis. Constipation receptors Receptors Receptors are proteins located either on the surface of or within a cell that can bind to signaling molecules known as ligands (e.g., hormones) and cause some type of response within the cell. Receptors:
Opioids can be characterized as:
The following table summarizes the stepwise approach recommended for treating pain Pain An unpleasant sensation induced by noxious stimuli which are detected by nerve endings of nociceptive neurons. Pain: Types and Pathways:
| Step | Recommended therapy |
|---|---|
| 1 | Nonopioid analgesics |
| 2 | Nonopioid analgesics + mild opioids (tramadol) |
| 3 | Nonopioid analgesics + strong opioids (morphine, hydromorphone, and oxycodone) |
| 4 | Invasive techniques such as epidural injection, peripheral local anesthesia Anesthesia A state characterized by loss of feeling or sensation. This depression of nerve function is usually the result of pharmacologic action and is induced to allow performance of surgery or other painful procedures. Anesthesiology: History and Basic Concepts, and ganglion block |
| Drug | MME |
|---|---|
| Codeine | 0.15 |
| Fentanyl transdermal (in mcg/hr) | 2.4 |
| Hydrocodone Hydrocodone Narcotic analgesic related to codeine, but more potent and more addicting by weight. It is used also as cough suppressant. Neonatal Abstinence Syndrome | 1 |
| Hydromorphone | 4 |
Methadone:
|
|
| Morphine | 1 |
| Oxycodone | 1.5 |
| Oxymorphone | 3 |
Absolute:
Relative:
Use with caution:
The following table compares the most common opioid Opioid Compounds with activity like opiate alkaloids, acting at opioid receptors. Properties include induction of analgesia or narcosis. Constipation antagonists:
| Medication | Mechanism | Indication | Side effects |
|---|---|---|---|
| Naloxone | Mu: competitive antagonist |
|
Opioid withdrawal Opioid withdrawal Opioid Use Disorder |
| Naltrexone | Competitive antagonist at mu, kappa, and delta receptors Receptors Receptors are proteins located either on the surface of or within a cell that can bind to signaling molecules known as ligands (e.g., hormones) and cause some type of response within the cell. Receptors |
|
|
| Methylnaltrexone | Mu: competitive antagonist in the GI tract (does not cross the blood–brain barrier Blood–Brain Barrier Meningitis in Children) | Opioid-induced constipation Constipation Constipation is common and may be due to a variety of causes. Constipation is generally defined as bowel movement frequency < 3 times per week. Patients who are constipated often strain to pass hard stools. The condition is classified as primary (also known as idiopathic or functional constipation) or secondary, and as acute or chronic. Constipation |
|
The following table compares and contrasts characteristics of the opioid Opioid Compounds with activity like opiate alkaloids, acting at opioid receptors. Properties include induction of analgesia or narcosis. Constipation analgesics:
| Medication and formulation | Receptor Receptor Receptors are proteins located either on the surface of or within a cell that can bind to signaling molecules known as ligands (e.g., hormones) and cause some type of response within the cell. Receptors effects | Pharmacokinetics Pharmacokinetics Pharmacokinetics is the science that analyzes how the human body interacts with a drug. Pharmacokinetics examines how the drug is absorbed, distributed, metabolized, and excreted by the body. Pharmacokinetics and Pharmacodynamics | Important facts |
|---|---|---|---|
| Opioid agonists Opioid Agonists Antidiarrheal Drugs | |||
| Morphine | Strong agonist |
|
Metabolite has potential neuroexcitation effects. |
| Fentanyl | Strong agonist |
|
Transdermal formulation for extended-release delivery |
| Hydromorphone | Strong agonist |
|
|
| Codeine | Moderate agonist |
|
Schedule III in combination with acetaminophen Acetaminophen Acetaminophen is an over-the-counter nonopioid analgesic and antipyretic medication and the most commonly used analgesic worldwide. Despite the widespread use of acetaminophen, its mechanism of action is not entirely understood. Acetaminophen |
| Oxycodone | Moderate agonist |
|
Frequently combined with acetaminophen Acetaminophen Acetaminophen is an over-the-counter nonopioid analgesic and antipyretic medication and the most commonly used analgesic worldwide. Despite the widespread use of acetaminophen, its mechanism of action is not entirely understood. Acetaminophen |
| Hydrocodone Hydrocodone Narcotic analgesic related to codeine, but more potent and more addicting by weight. It is used also as cough suppressant. Neonatal Abstinence Syndrome | Moderate agonist |
|
Frequently combined with acetaminophen Acetaminophen Acetaminophen is an over-the-counter nonopioid analgesic and antipyretic medication and the most commonly used analgesic worldwide. Despite the widespread use of acetaminophen, its mechanism of action is not entirely understood. Acetaminophen |
| Tramadol |
|
|
|
| Methadone |
|
|
|
| Agonist–antagonists | |||
| Nalbuphine |
|
|
|
| Buprenorphine |
|
|
|