Pain Pain An unpleasant sensation induced by noxious stimuli which are detected by nerve endings of nociceptive neurons. Pain: Types and Pathways is defined as an unpleasant sensory Sensory Neurons which conduct nerve impulses to the central nervous system. Nervous System: Histology and emotional experience associated with actual or potential tissue damage. Pain Pain An unpleasant sensation induced by noxious stimuli which are detected by nerve endings of nociceptive neurons. Pain: Types and Pathways is a subjective experience. Acute pain Pain An unpleasant sensation induced by noxious stimuli which are detected by nerve endings of nociceptive neurons. Pain: Types and Pathways lasts < 3 months and typically has a specific, identifiable cause. Chronic pain Pain An unpleasant sensation induced by noxious stimuli which are detected by nerve endings of nociceptive neurons. Pain: Types and Pathways lasts > 3 months and may exist in the absence of tissue damage or after healing would have been expected to occur. Pain Pain An unpleasant sensation induced by noxious stimuli which are detected by nerve endings of nociceptive neurons. Pain: Types and Pathways management involves a combination of addressing underlying causes and using a systematic approach tailored to the clinical scenario.
Last updated: May 17, 2024
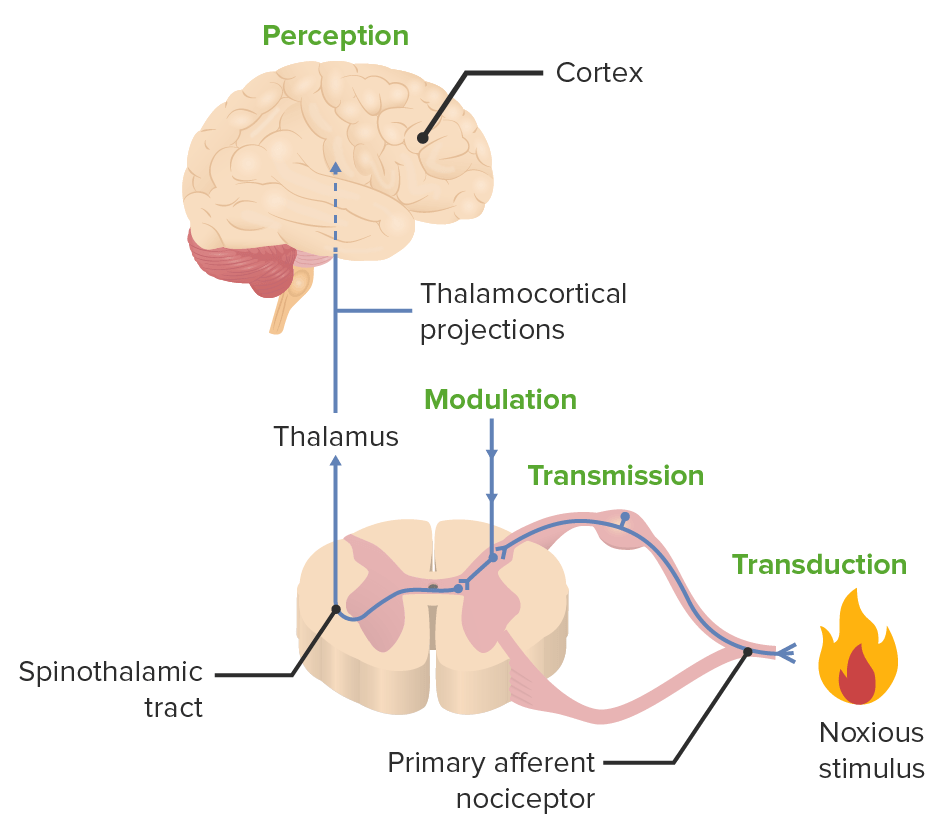
The 4 processes that make up nociception:
transduction, transmission, modulation, and perception
| Type | Duration | Characteristic |
|---|---|---|
| Acute pain Pain An unpleasant sensation induced by noxious stimuli which are detected by nerve endings of nociceptive neurons. Pain: Types and Pathways | < 3 months |
|
| Chronic pain Pain An unpleasant sensation induced by noxious stimuli which are detected by nerve endings of nociceptive neurons. Pain: Types and Pathways | > 3 months |
|
| Type | Duration | Characteristic |
|---|---|---|
| Nociceptive pain Nociceptive pain Dull or sharp aching pain caused by stimulated nociceptors due to tissue injury, inflammation or diseases. It can be divided into somatic or tissue pain and visceral pain. Pain: Types and Pathways | Typically acute |
|
| Neuropathic pain Neuropathic pain Caused by lesion or disease affecting the nervous system (PNS or CNS). Pain: Types and Pathways | Variable Variable Variables represent information about something that can change. The design of the measurement scales, or of the methods for obtaining information, will determine the data gathered and the characteristics of that data. As a result, a variable can be qualitative or quantitative, and may be further classified into subgroups. Types of Variables, often chronic |
|
| Nociplastic pain Pain An unpleasant sensation induced by noxious stimuli which are detected by nerve endings of nociceptive neurons. Pain: Types and Pathways (recently defined) | Usually chronic or intermittent |
|
The following principles are recommended by the World Health Organization (WHO) as a basis for the treatment of chronic pain Pain An unpleasant sensation induced by noxious stimuli which are detected by nerve endings of nociceptive neurons. Pain: Types and Pathways:
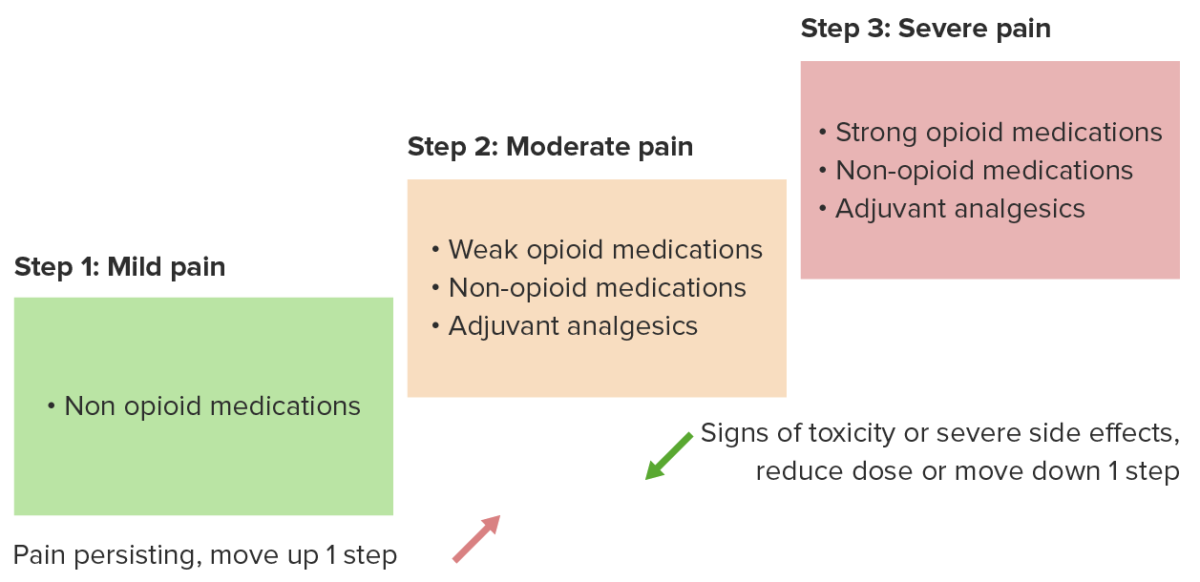
Analgesic ladder for cancer pain
Image by Lecturio.| Nociceptive pain Nociceptive pain Dull or sharp aching pain caused by stimulated nociceptors due to tissue injury, inflammation or diseases. It can be divided into somatic or tissue pain and visceral pain. Pain: Types and Pathways |
|
|---|---|
| Neuropathic pain Neuropathic pain Caused by lesion or disease affecting the nervous system (PNS or CNS). Pain: Types and Pathways | If possible, identify and correct mechanical
nerve compression
Nerve Compression
Brachial Plexus Injuries via
physical therapy
Physical Therapy
Becker Muscular Dystrophy and/or surgery. First-line:
|
| Non-pharmacological treatments | To be used as a first-line measure or as an adjunct in multimodal
pain
Pain
An unpleasant sensation induced by noxious stimuli which are detected by nerve endings of nociceptive neurons.
Pain: Types and Pathways management:
|