Carbonic anhydrase inhibitors (CAIs) block the carbonic anhydrase enzymes Enzymes Enzymes are complex protein biocatalysts that accelerate chemical reactions without being consumed by them. Due to the body's constant metabolic needs, the absence of enzymes would make life unsustainable, as reactions would occur too slowly without these molecules. Basics of Enzymes in the proximal convoluted tubule Proximal convoluted tubule The renal tubule portion that extends from the bowman capsule in the kidney cortex into the kidney medulla. The proximal tubule consists of a convoluted proximal segment in the cortex, and a distal straight segment descending into the medulla where it forms the u-shaped loop of henle. Osmotic Diuretics, inhibiting the reabsorption of sodium Sodium A member of the alkali group of metals. It has the atomic symbol na, atomic number 11, and atomic weight 23. Hyponatremia bicarbonate Bicarbonate Inorganic salts that contain the -HCO3 radical. They are an important factor in determining the ph of the blood and the concentration of bicarbonate ions is regulated by the kidney. Levels in the blood are an index of the alkali reserve or buffering capacity. Electrolytes (NaHCO3), which results in diuresis and metabolic acidosis Acidosis A pathologic condition of acid accumulation or depletion of base in the body. The two main types are respiratory acidosis and metabolic acidosis, due to metabolic acid build up. Respiratory Acidosis. Carbonic anhydrase inhibitors also block the carbonic anhydrase present in the eyes and glial cells Glial Cells The non-neuronal cells of the nervous system. They not only provide physical support, but also respond to injury, regulate the ionic and chemical composition of the extracellular milieu, participate in the blood-brain barrier and blood-retinal barrier, form the myelin insulation of nervous pathways, guide neuronal migration during development, and exchange metabolites with neurons. Neuroglia have high-affinity transmitter uptake systems, voltage-dependent and transmitter-gated ion channels, and can release transmitters, but their role in signaling (as in many other functions) is unclear. Nervous System: Histology, resulting in decreased aqueous humor Humor Defense Mechanisms and CSF production, respectively. Acetazolamide is the prototypical CAI. Carbonic anhydrase inhibitors are mainly used for the treatment of altitude sickness Altitude Sickness Altitude sickness refers to a spectrum of symptoms caused by physiological changes in the human body at altitudes above 2,500 m. Altitude sickness includes acute mountain sickness (AMS), high-altitude cerebral edema (HACE), and high-altitude pulmonary edema (HAPE). Altitude Sickness, edema Edema Edema is a condition in which excess serous fluid accumulates in the body cavity or interstitial space of connective tissues. Edema is a symptom observed in several medical conditions. It can be categorized into 2 types, namely, peripheral (in the extremities) and internal (in an organ or body cavity). Edema in patients Patients Individuals participating in the health care system for the purpose of receiving therapeutic, diagnostic, or preventive procedures. Clinician–Patient Relationship with metabolic alkalosis Alkalosis A pathological condition that removes acid or adds base to the body fluids. Respiratory Alkalosis, glaucoma Glaucoma Glaucoma is an optic neuropathy characterized by typical visual field defects and optic nerve atrophy seen as optic disc cupping on examination. The acute form of glaucoma is a medical emergency. Glaucoma is often, but not always, caused by increased intraocular pressure (IOP). Glaucoma, and, sometimes, as an adjuvant Adjuvant Substances that augment, stimulate, activate, potentiate, or modulate the immune response at either the cellular or humoral level. The classical agents (freund's adjuvant, bcg, corynebacterium parvum, et al.) contain bacterial antigens. Some are endogenous (e.g., histamine, interferon, transfer factor, tuftsin, interleukin-1). Their mode of action is either non-specific, resulting in increased immune responsiveness to a wide variety of antigens, or antigen-specific, i.e., affecting a restricted type of immune response to a narrow group of antigens. The therapeutic efficacy of many biological response modifiers is related to their antigen-specific immunoadjuvanticity. Vaccination treatment for certain types of epilepsies and increased intracranial pressure Intracranial Pressure Idiopathic Intracranial Hypertension. Carbonic anhydrase inhibitors are not used for the treatment of hypertension Hypertension Hypertension, or high blood pressure, is a common disease that manifests as elevated systemic arterial pressures. Hypertension is most often asymptomatic and is found incidentally as part of a routine physical examination or during triage for an unrelated medical encounter. Hypertension.
Last updated: Jul 7, 2023
Carbonic anhydrase inhibitors (CAIs) are diuretics Diuretics Agents that promote the excretion of urine through their effects on kidney function. Heart Failure and Angina Medication that block the carbonic anhydrase enzymes Enzymes Enzymes are complex protein biocatalysts that accelerate chemical reactions without being consumed by them. Due to the body’s constant metabolic needs, the absence of enzymes would make life unsustainable, as reactions would occur too slowly without these molecules. Basics of Enzymes.
Acetazolamide is a sulfonamide Sulfonamide The sulfonamides are a class of antimicrobial drugs inhibiting folic acid synthesize in pathogens. The prototypical drug in the class is sulfamethoxazole. Although not technically sulfonamides, trimethoprim, dapsone, and pyrimethamine are also important antimicrobial agents inhibiting folic acid synthesis. The agents are often combined with sulfonamides, resulting in a synergistic effect. Sulfonamides and Trimethoprim.
Chemical structure of acetazolamide
Image: “Acetazolamide” by Ayacop. License: Public DomainBicarbonate Bicarbonate Inorganic salts that contain the -HCO3 radical. They are an important factor in determining the ph of the blood and the concentration of bicarbonate ions is regulated by the kidney. Levels in the blood are an index of the alkali reserve or buffering capacity. Electrolytes cannot be directly reabsorbed by the kidneys Kidneys The kidneys are a pair of bean-shaped organs located retroperitoneally against the posterior wall of the abdomen on either side of the spine. As part of the urinary tract, the kidneys are responsible for blood filtration and excretion of water-soluble waste in the urine. Kidneys: Anatomy; it must be converted into CO2 in the lumen and then reconverted to HCO3– once inside the cell. This reaction occurs via the following processes:
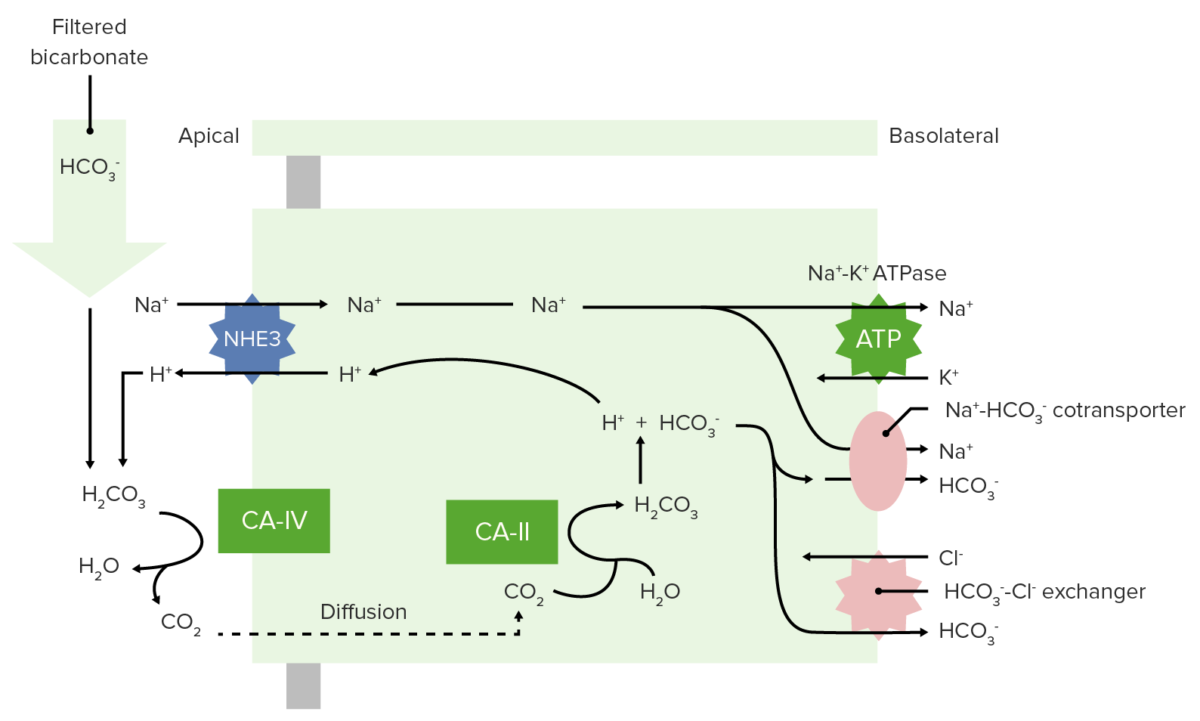
Bicarbonate reabsorption in the proximal tubule
NHE3: Na+-H+ ion exchanger 3
CA-IV: carbonic anhydrase IV
CA-II: carbonic anhydrase II
Use of CAI’s results in:
| Drug | Absorption Absorption Absorption involves the uptake of nutrient molecules and their transfer from the lumen of the GI tract across the enterocytes and into the interstitial space, where they can be taken up in the venous or lymphatic circulation. Digestion and Absorption | Distribution | Metabolism | Excretion |
|---|---|---|---|---|
| Acetazolamide |
|
|
Not metabolized |
|
| Methazolamide |
|
|
Slowly within the GI tract |
|
Carbonic anhydrase inhibitors should be used with caution in the following populations:
Some of the other most common diuretics Diuretics Agents that promote the excretion of urine through their effects on kidney function. Heart Failure and Angina Medication include thiazide Thiazide Heterocyclic compounds with sulfur and nitrogen in the ring. This term commonly refers to the benzothiadiazines that inhibit sodium-potassium-chloride symporters and are used as diuretics. Hyponatremia diuretics Diuretics Agents that promote the excretion of urine through their effects on kidney function. Heart Failure and Angina Medication (e.g., hydrochlorothiazide Hydrochlorothiazide A thiazide diuretic often considered the prototypical member of this class. It reduces the reabsorption of electrolytes from the renal tubules. This results in increased excretion of water and electrolytes, including sodium, potassium, chloride, and magnesium. It is used in the treatment of several disorders including edema, hypertension, diabetes insipidus, and hypoparathyroidism. Thiazide Diuretics), loop diuretics Diuretics Agents that promote the excretion of urine through their effects on kidney function. Heart Failure and Angina Medication (e.g., furosemide Furosemide A benzoic-sulfonamide-furan. It is a diuretic with fast onset and short duration that is used for edema and chronic renal insufficiency. Loop Diuretics), K+-sparing diuretics Diuretics Agents that promote the excretion of urine through their effects on kidney function. Heart Failure and Angina Medication (e.g., spironolactone Spironolactone A potassium sparing diuretic that acts by antagonism of aldosterone in the distal renal tubules. It is used mainly in the treatment of refractory edema in patients with congestive heart failure, nephrotic syndrome, or hepatic cirrhosis. Its effects on the endocrine system are utilized in the treatments of hirsutism and acne but they can lead to adverse effects. Potassium-sparing Diuretics), and osmotic diuretics Diuretics Agents that promote the excretion of urine through their effects on kidney function. Heart Failure and Angina Medication (e.g., mannitol Mannitol A diuretic and renal diagnostic aid related to sorbitol. It has little significant energy value as it is largely eliminated from the body before any metabolism can take place. It can be used to treat oliguria associated with kidney failure or other manifestations of inadequate renal function and has been used for determination of glomerular filtration rate. Mannitol is also commonly used as a research tool in cell biological studies, usually to control osmolarity. Osmotic Diuretics).
| Medication | Mechanism | Physiologic effect | Indication |
|---|---|---|---|
| Thiazide Thiazide Heterocyclic compounds with sulfur and nitrogen in the ring. This term commonly refers to the benzothiadiazines that inhibit sodium-potassium-chloride symporters and are used as diuretics. Hyponatremia diuretic: Hydrochlorothiazide Hydrochlorothiazide A thiazide diuretic often considered the prototypical member of this class. It reduces the reabsorption of electrolytes from the renal tubules. This results in increased excretion of water and electrolytes, including sodium, potassium, chloride, and magnesium. It is used in the treatment of several disorders including edema, hypertension, diabetes insipidus, and hypoparathyroidism. Thiazide Diuretics | ↓ Reabsorption of NaCl in the DCT through the inhibition of Na+/Cl– cotransporter |
|
|
| Loop diuretic: Furosemide Furosemide A benzoic-sulfonamide-furan. It is a diuretic with fast onset and short duration that is used for edema and chronic renal insufficiency. Loop Diuretics | Inhibits the luminal Na+/K+/Cl– cotransporter in the thick ascending limb Thick ascending limb Renal Sodium and Water Regulation of the loop of Henle Loop of Henle The U-shaped portion of the renal tubule in the kidney medulla, consisting of a descending limb and an ascending limb. It is situated between the proximal kidney tubule and the distal kidney tubule. Tubular System |
|
|
| Potassium-sparing diuretic: Spironolactone Spironolactone A potassium sparing diuretic that acts by antagonism of aldosterone in the distal renal tubules. It is used mainly in the treatment of refractory edema in patients with congestive heart failure, nephrotic syndrome, or hepatic cirrhosis. Its effects on the endocrine system are utilized in the treatments of hirsutism and acne but they can lead to adverse effects. Potassium-sparing Diuretics |
|
|
|
| Carbonic anhydrase inhibitor Carbonic Anhydrase Inhibitor Glaucoma: Acetazolamide | Inhibits both the hydration of CO2 in the PCT epithelial cells and the dehydration Dehydration The condition that results from excessive loss of water from a living organism. Volume Depletion and Dehydration of H2CO3 in the PCT lumen; results in ↑ HCO3– and Na+ excretion |
|
|
| Osmotic diuretics Diuretics Agents that promote the excretion of urine through their effects on kidney function. Heart Failure and Angina Medication: Mannitol Mannitol A diuretic and renal diagnostic aid related to sorbitol. It has little significant energy value as it is largely eliminated from the body before any metabolism can take place. It can be used to treat oliguria associated with kidney failure or other manifestations of inadequate renal function and has been used for determination of glomerular filtration rate. Mannitol is also commonly used as a research tool in cell biological studies, usually to control osmolarity. Osmotic Diuretics | ↑ Osmotic pressure Osmotic pressure The pressure required to prevent the passage of solvent through a semipermeable membrane that separates a pure solvent from a solution of the solvent and solute or that separates different concentrations of a solution. It is proportional to the osmolality of the solution. Intravenous Fluids in the glomerular filtrate → ↑ tubular fluid and prevents water reabsorption |
|
|
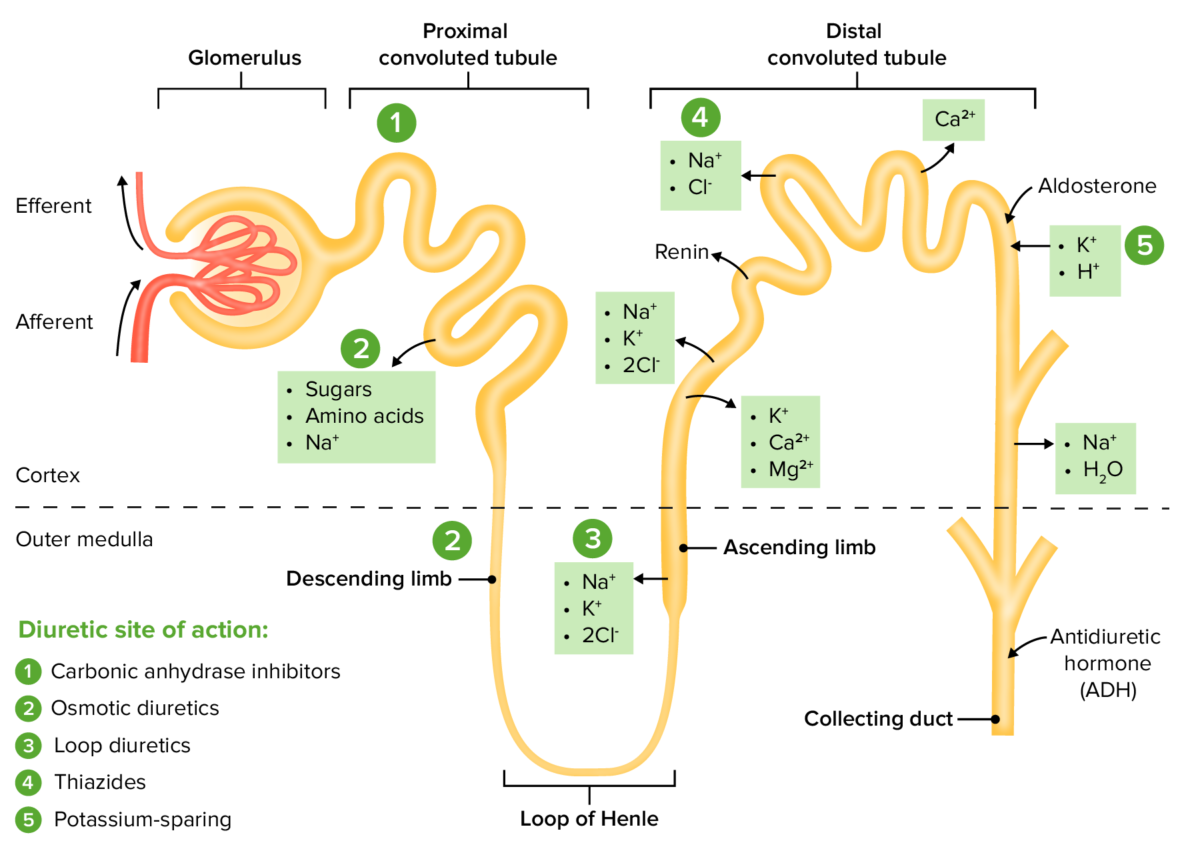
The sites of action within the nephron for the diuretic drug classes
Image by Lecturio. License: CC BY-NC-SA 4.0