The renal system is responsible for eliminating the daily load of non-volatile acids Acids Chemical compounds which yield hydrogen ions or protons when dissolved in water, whose hydrogen can be replaced by metals or basic radicals, or which react with bases to form salts and water (neutralization). An extension of the term includes substances dissolved in media other than water. Acid-Base Balance, which is approximately 70 millimoles per day. This daily load comes primarily from anaerobic metabolism, absorption Absorption Absorption involves the uptake of nutrient molecules and their transfer from the lumen of the GI tract across the enterocytes and into the interstitial space, where they can be taken up in the venous or lymphatic circulation. Digestion and Absorption of acids Acids Chemical compounds which yield hydrogen ions or protons when dissolved in water, whose hydrogen can be replaced by metals or basic radicals, or which react with bases to form salts and water (neutralization). An extension of the term includes substances dissolved in media other than water. Acid-Base Balance, and excretion of base from the GI system. Metabolic alkalosis Alkalosis A pathological condition that removes acid or adds base to the body fluids. Respiratory Alkalosis develops when there is an increase in serum HCO3- levels. Metabolic alkalosis Alkalosis A pathological condition that removes acid or adds base to the body fluids. Respiratory Alkalosis also occurs when there is an increased loss of acid, either renally or through the upper GI tract (e.g., vomiting Vomiting The forcible expulsion of the contents of the stomach through the mouth. Hypokalemia), increased intake of HCO3-, or a reduced ability to secrete HCO3- when needed. Respiratory compensation Compensation Respiratory Acidosis occurs very quickly (within minutes) and mitigates changes in pH pH The quantitative measurement of the acidity or basicity of a solution. Acid-Base Balance from the primary metabolic disorder. Management is aimed at correcting the underlying etiology.
Last updated: Jul 5, 2023
Metabolic alkalosis Alkalosis A pathological condition that removes acid or adds base to the body fluids. Respiratory Alkalosis is the process that results in the loss of hydrogen ions (H+) or the gain of HCO3–. In primary metabolic alkalosis Alkalosis A pathological condition that removes acid or adds base to the body fluids. Respiratory Alkalosis, arterial blood gas Arterial blood gas Respiratory Alkalosis will show:
Acid-base disorders are classified according to the primary disturbance (respiratory or metabolic) and the presence or absence of compensation Compensation Respiratory Acidosis.
Consider pH pH The quantitative measurement of the acidity or basicity of a solution. Acid-Base Balance, PCO2, and HCO3– to determine the primary disturbance.
When a patient develops acidosis Acidosis A pathologic condition of acid accumulation or depletion of base in the body. The two main types are respiratory acidosis and metabolic acidosis, due to metabolic acid build up. Respiratory Acidosis or alkalosis Alkalosis A pathological condition that removes acid or adds base to the body fluids. Respiratory Alkalosis, the body will try to compensate. Oftentimes, compensation Compensation Respiratory Acidosis will result in normal pH pH The quantitative measurement of the acidity or basicity of a solution. Acid-Base Balance.
The kidneys Kidneys The kidneys are a pair of bean-shaped organs located retroperitoneally against the posterior wall of the abdomen on either side of the spine. As part of the urinary tract, the kidneys are responsible for blood filtration and excretion of water-soluble waste in the urine. Kidneys: Anatomy have an extensive ability to upregulate HCO3– elimination Elimination The initial damage and destruction of tumor cells by innate and adaptive immunity. Completion of the phase means no cancer growth. Cancer Immunotherapy; therefore, a pathological process needs to be present for metabolic alkalosis Alkalosis A pathological condition that removes acid or adds base to the body fluids. Respiratory Alkalosis to be maintained. Alkalosis Alkalosis A pathological condition that removes acid or adds base to the body fluids. Respiratory Alkalosis is maintained via decreased HCO3– excretion or increased HCO3– reabsorption.
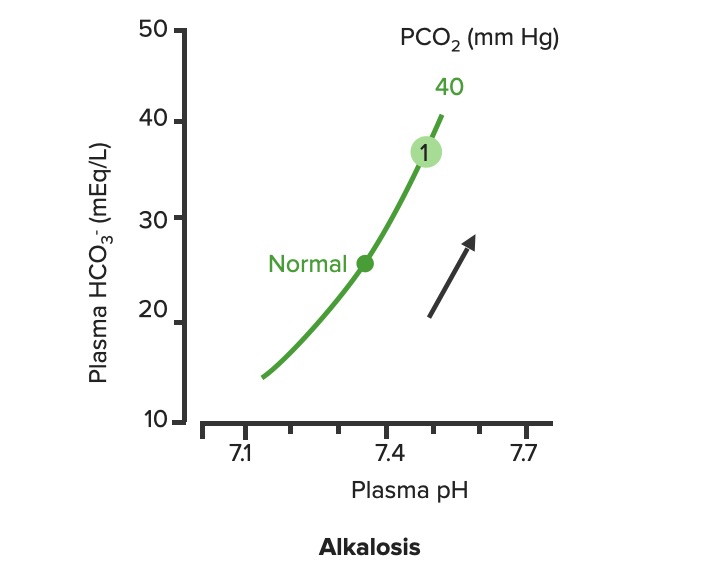
Relationship between plasma pH and plasma HCO3– in uncompensated metabolic alkalosis (1). Notice how the increase of HCO3– moves along the PCO2.
Image by Lecturio.Compensatory respiratory acidosis Acidosis A pathologic condition of acid accumulation or depletion of base in the body. The two main types are respiratory acidosis and metabolic acidosis, due to metabolic acid build up. Respiratory Acidosis occurs in response to metabolic alkalosis Alkalosis A pathological condition that removes acid or adds base to the body fluids. Respiratory Alkalosis.
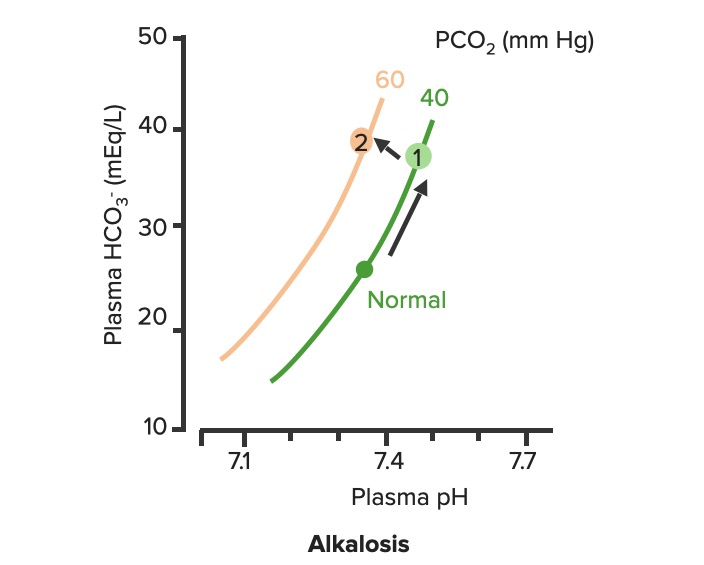
Respiratory compensation of metabolic alkalosis:
As PCO2 increases, the curve shifts up and to the left along the “blood-buffer line” (2). As the curve shifts, the pH decreases towards normal.
The clinical presentation is dependent on the underlying etiology. Symptoms may include:

Trousseau sign:
Trousseau sign is induced by inflating the blood pressure cuff above systolic pressure for 3 minutes. The sign is demonstrated by an adducted thumb, flexed metacarpophalangeal joints, extended interphalangeal joints, and flexed wrist.
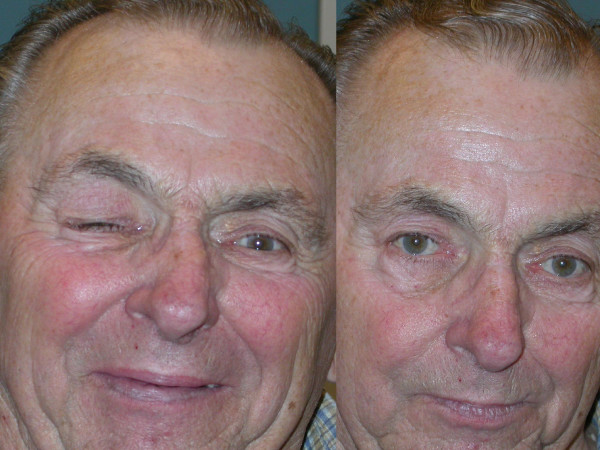
Chvostek sign:
Chvostek sign is Induced by tapping on the facial nerve of the cheek, which leads to twitching of the mouth musculature.
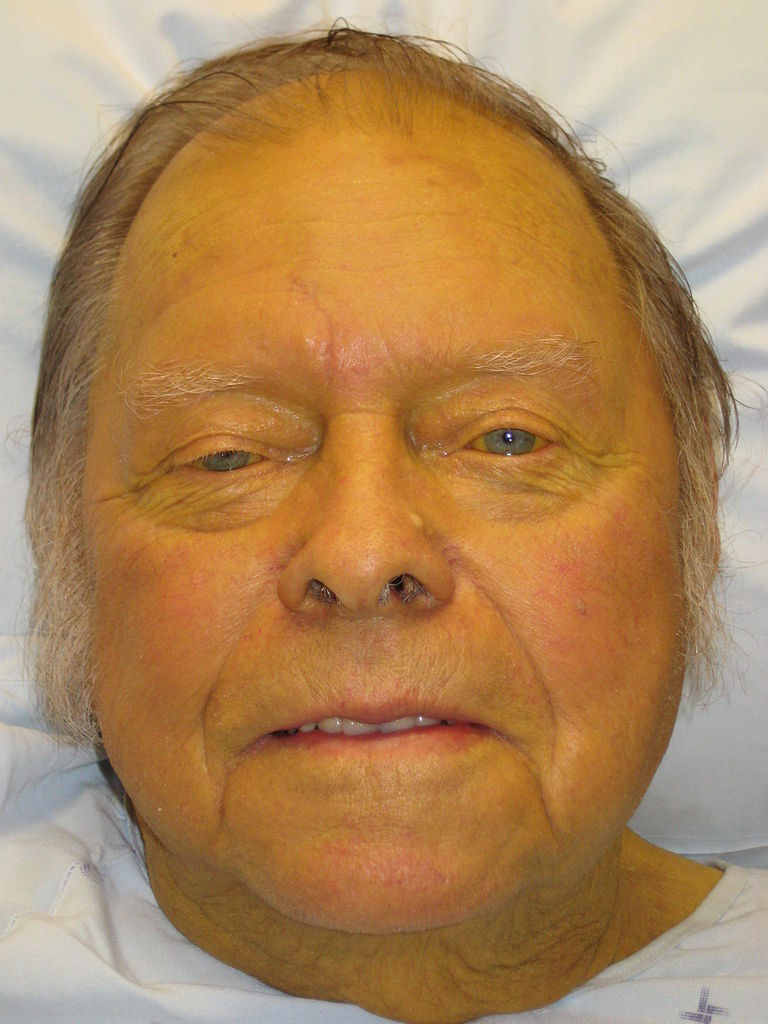
Jaundice: sign observed in cirrhosis presenting with yellow discoloration of the skin and sclera
Image: “Jaundice08” by James Heilman, MD. License: CC BY 3.0The etiology of metabolic alkalosis Alkalosis A pathological condition that removes acid or adds base to the body fluids. Respiratory Alkalosis is usually ascertainable from the history alone. Urine Cl– can be helpful in cases in which the patient is reluctant to provide a full history (e.g., self-induced vomiting Vomiting The forcible expulsion of the contents of the stomach through the mouth. Hypokalemia in eating disorders) or for less common etiologies (e.g., Conn, Bartter, and Gitelman syndromes).
Urine chloride Chloride Inorganic compounds derived from hydrochloric acid that contain the Cl- ion. Electrolytes:
Other tests:
Treatment is aimed at the underlying etiology.